Pharmaceutical compound
 | |
| Clinical data | |
|---|---|
| Trade names | Manyper, Caldine, etc. |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C35H38N4O6 |
| Molar mass | 610.711 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Manidipine is a calcium channel blocker (dihydropyridine type) that is used clinically as an antihypertensive.
It was patented in 1982 and approved for medical use in 1990.
Synthesis
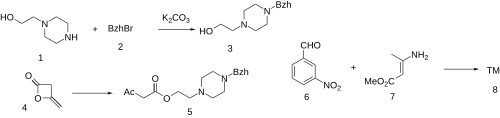
The alkylation between N-(2-hydroxyethyl)piperazine (1) and Benzhydryl Bromide (2) gives 2-(4-benzhydrylpiperazin-1-yl)ethanol (3). The reaction with Diketene (4) gives 2-(4-benzhydryl-1-piperazinyl)ethyl acetoacetate (5). The reaction with 3-nitrobenzaldehyde (6) and Methyl 3-aminocrotonate (7) completed the synthesis of Manidipine (8).
References
- Cheer SM, McClellan K (2001). "Manidipine: a review of its use in hypertension". Drugs. 61 (12): 1777–1799. doi:10.2165/00003495-200161120-00010. PMID 11693466. S2CID 260814599. Archived from the original on 2013-01-17. Retrieved 2009-06-20.
- McKeage K, Scott LJ (2004). "Manidipine: a review of its use in the management of hypertension". Drugs. 64 (17): 1923–1940. doi:10.2165/00003495-200464170-00011. PMID 15329044. S2CID 195689527. Archived from the original on 2013-01-16. Retrieved 2009-06-20.
- Roca-Cusachs A, Triposkiadis F (2005). "Antihypertensive effect of manidipine". Drugs. 65 (Suppl 2): 11–19. doi:10.2165/00003495-200565002-00003. PMID 16398058. S2CID 25854593. Archived from the original on 2013-01-16. Retrieved 2009-06-20.
- Otero ML (2007). "Manidipine-delapril combination in the management of hypertension". Vascular Health and Risk Management. 3 (3): 255–263. PMC 2293964. PMID 17703633.
- Mizuno K, Haga H, Takahashi M, Fukuchi S (August 1992). "Evaluation of manidipine hydrochloride, a new calcium antagonist, in the treatment of hypertensive patients with renal disorders". Current Therapeutic Research. 52 (2): 248–253. doi:10.1016/S0011-393X(05)80475-8.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 465. ISBN 9783527607495.
- Meguro, Kanji; Aizawa, Masahiro; Sohda, Takashi; Kawamatsu, Yutaka; Nagaoka, Akinobu (1985). "New 1,4-dihydropyridine derivatives with potent and long-lasting hypotensive effect.". CHEMICAL & PHARMACEUTICAL BULLETIN. 33 (9): 3787–3797. ISSN 0009-2363. doi:10.1248/cpb.33.3787.
- EP0094159 idem Kanji Meguro & Akinobu Nagaoka, US4892875 (1990 to Takeda Pharmaceutical Co Ltd).
- Dharmaraj Ramachandra Rao, Rajendra Narayanrao Kankan, Maruti Ganpati Ghagare, WO20110203954 (2011 to Cipla Limited, Curtis, Philip Anthony).
- 刘玉海, et al. CN105924382 (2018).
- 金晓峰, et al. CN102875451 (2014 to CHANGZHOU PHARMACEUTICAL FACTORY CO LTD).
- 刘忠春, CN107337632 (2017).
- 谷志勇, et al. CN104292150 (2015).
- , CN103351362 (2013 to).
- http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZHOU200402000.htm
| Piperazines | |
|---|---|
| Simple piperazines (no additional rings) | |
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines | |
| Pyridinylpiperazines | |
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized | |
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |