 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
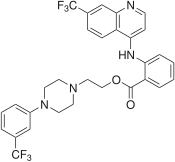
| Formula | C30H26F6N4O2 |
| Molar mass | 588.554 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Antrafenine (Stakane) is a phenylpiperazine derivative drug invented in 1979. It acts as an analgesic and anti-inflammatory drug with similar efficacy to naproxen, but is not widely used as it has largely been replaced by newer drugs.
Synthesis

Method E: The reaction between 2--1-piperazinyl]ethanol (1) and Isatoic anhydride (2) goes on to give 4-(3-(Trifluoromethyl)phenyl)piperazine-1-ethyl 2-aminobenzoate (3).
Method G: Alkylation with 4-chloro-7-(trifluoromethyl)quinoline (4) completed the synthesis of antrafenine (5).
See also
References
- ^ Manoury PM, Dumas AP, Najer H, Branceni D, Prouteau M, Lefevre-Borg FM (May 1979). "Synthesis and analgesic activities of some (4-substituted phenyl-1-piperazinyl)alkyl 2-aminobenzoates and 2-aminonicotinates". Journal of Medicinal Chemistry. 22 (5): 554–9. doi:10.1021/jm00191a017. PMID 458805.
- Leatham PA, Bird HA, Wright V, Seymour D, Gordon A (1983). "A double blind study of antrafenine, naproxen and placebo in osteoarthrosis". European Journal of Rheumatology and Inflammation. 6 (2): 209–11. PMID 6673985.
- Don Pierre Rene Lucien Giudicelli, et al. U.S. patent 4,017,623 (1977 to Synthelabo SA).
- Don Pierre Rene Lucien Giudicelli, et al. U.S. patent 3,935,229 (1976 to Synthelabo SA).
- Don Pierre Rene Lucien Giudicelli, et al. U.S. patent 3,953,449 (1976 to Synthelabo SA).
| Non-steroidal anti-inflammatory drugs (NSAIDs) (primarily M01A and M02A, also N02BA) | |
|---|---|
| pyrazolones / pyrazolidines | |
| salicylates | |
| acetic acid derivatives and related substances | |
| oxicams | |
| propionic acid derivatives (profens) |
|
| n-arylanthranilic acids (fenamates) | |
| COX-2 inhibitors (coxibs) | |
| other | |
| NSAID combinations | |
| Key: underline indicates initially developed first-in-class compound of specific group; WHO-Essential Medicines; withdrawn drugs; veterinary use. | |
| Prostanoid signaling modulators | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor (ligands) |
| ||||||||||||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||||||||||||
| Others | |||||||||||||||||||||||||||
| Piperazines | |
|---|---|
| Simple piperazines (no additional rings) | |
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines | |
| Pyridinylpiperazines | |
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized | |
This drug article relating to the musculoskeletal system is a stub. You can help Misplaced Pages by expanding it. |