 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
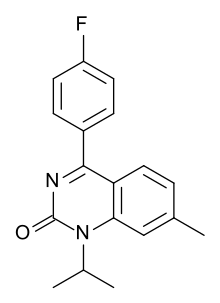
| Formula | C18H17FN2O |
| Molar mass | 296.345 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Fluproquazone (trade name Tormosyl, RF 46-790 ) was a quinazolinone derivative with potent analgesic, antipyretic, and anti-inflammatory effects discovered by Sandoz. It was withdrawn during development due to liver toxicity.
References
- Haanaes HR, Benterud UJ, Skoglund LA (November 1986). "RF 46-790 versus paracetamol: effect on post-operative pain". International Journal of Clinical Pharmacology, Therapy, and Toxicology. 24 (11): 598–601. PMID 3491794.
- Mohing W, Suckert R, Lataste X (1981). "Comparative study of fluproquazone in the management of post-operative pain". Arzneimittel-Forschung. 31 (5a): 918–20. PMID 6973986.
- Wheatley D (May 1982). "Analgesic properties of fluproquazone". Rheumatology and Rehabilitation. 21 (2): 98–100. doi:10.1093/rheumatology/21.2.98. PMID 7043713.
- Fankhauser S, Laube W, Marti HR, Schultheiss HR, Vögtlin J, von Graffenried B (1981). "Antipyretic activity of fluproquazone in man". Arzneimittel-Forschung. 31 (5a): 934–5. PMID 6973990.
- Lewis JH, Stine JG (2013). "Nonsteroidal Antiinflammatory Drugs and Leukotriene Receptor Antagonists". In Kaplowitz N, DeLeve LD (eds.). Drug-induced Liver Disease (third ed.). Elsevier Inc. ISBN 9780123878175.
- Zimmerman HJ (1999). Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. Lippincott Williams & Wilkins. ISBN 9780781719520.
| Non-steroidal anti-inflammatory drugs (NSAIDs) (primarily M01A and M02A, also N02BA) | |
|---|---|
| pyrazolones / pyrazolidines | |
| salicylates | |
| acetic acid derivatives and related substances | |
| oxicams | |
| propionic acid derivatives (profens) |
|
| n-arylanthranilic acids (fenamates) | |
| COX-2 inhibitors (coxibs) | |
| other | |
| NSAID combinations | |
| Key: underline indicates initially developed first-in-class compound of specific group; WHO-Essential Medicines; withdrawn drugs; veterinary use. | |
This drug article relating to the musculoskeletal system is a stub. You can help Misplaced Pages by expanding it. |