 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
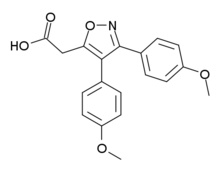
| Formula | C19H17NO5 |
| Molar mass | 339.347 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Mofezolac (INN), sold under the name Disopain in Japan, is a nonsteroidal anti-inflammatory drug (NSAID) used for its analgesic and anti-inflammatory actions. It is often prescribed for rheumatoid arthritis, lower back pain, frozen shoulder, and pain management after surgery or trauma. It is also being investigated for potential use in the treatment of neuroinflammation.
Common side effects include abdominal pain, stomach discomfort, nausea, sleepiness, itch, hives, rash, erythema, and edema. Serious side effects include peptic ulcers, gastrointestinal bleeding, asthma attack, jaundice, acute liver failure, and thrombocytopenia. Use is not recommended during pregnancy or breastfeeding.
Mofezolac acts via selective inhibition of the cyclooxygenase COX-1 and consequent suppression of prostaglandin synthesis. It is the most potent and selective reversible COX-1 inhibitor. Studies of ovine COX-1 in complex with mofezolac indicate that the drug forms a combination of electrostatic, H-bond, hydrophobic, and van der Waals contacts with the enzyme active site channel, contributing to mofezolac's high binding affinity.
Mofezolac belongs to the class of isoxazoles and is a substrate of CYP2C9.
It is manufactured and marketed by Nipro ES Pharma Co., Ltd.
References
- ^ "Kusuri-no-Shiori (Drug Information Sheet)". RAD-AR Council, Japan. June 2018. Retrieved 2021-10-18.
- Calvello R, Panaro MA, Carbone ML, Cianciulli A, Perrone MG, Vitale P, et al. (January 2012). "Novel selective COX-1 inhibitors suppress neuroinflammatory mediators in LPS-stimulated N13 microglial cells". Pharmacological Research. 65 (1): 137–148. doi:10.1016/j.phrs.2011.09.009. hdl:11586/117804. PMID 22001217.
- Calvello R, Lofrumento DD, Perrone MG, Cianciulli A, Salvatore R, Vitale P, et al. (2017). "Highly Selective Cyclooxygenase-1 Inhibitors P6 and Mofezolac Counteract Inflammatory State both In Vitro and In Vivo Models of Neuroinflammation". Frontiers in Neurology. 8: 251. doi:10.3389/fneur.2017.00251. PMC 5465243. PMID 28649222.
- "Pharmaceuticals and Medical Devices Safety Information" (381). Translated by Pharmaceuticals and Medical Devices Agency. Japan Ministry of Health, Labour and Welfare. March 2021.
{{cite journal}}: Cite journal requires|journal=(help) - Ono N, Yamamoto N, Sunami A, Yamasaki Y, Miyake H (February 1990). "[Pharmacological profile of mofezolac, a new non-steroidal analgesic anti-inflammatory drug]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 95 (2): 63–81. doi:10.1254/fpj.95.2_63. PMID 2109726.
- Pati ML, Vitale P, Ferorelli S, Iaselli M, Miciaccia M, Boccarelli A, et al. (February 2019). "Translational impact of novel widely pharmacological characterized mofezolac-derived COX-1 inhibitors combined with bortezomib on human multiple myeloma cell lines viability". European Journal of Medicinal Chemistry. 164: 59–76. doi:10.1016/j.ejmech.2018.12.029. hdl:11586/227733. PMID 30590258. S2CID 58648199.
- Cingolani G, Panella A, Perrone MG, Vitale P, Di Mauro G, Fortuna CG, et al. (September 2017). "Structural basis for selective inhibition of Cyclooxygenase-1 (COX-1) by diarylisoxazoles mofezolac and 3-(5-chlorofuran-2-yl)-5-methyl-4-phenylisoxazole (P6)". European Journal of Medicinal Chemistry. 138: 661–668. doi:10.1016/j.ejmech.2017.06.045. PMC 5992922. PMID 28710965.
- "DRUG: Mofezolac". KEGG. Retrieved 2021-10-18.
| Non-steroidal anti-inflammatory drugs (NSAIDs) (primarily M01A and M02A, also N02BA) | |
|---|---|
| pyrazolones / pyrazolidines | |
| salicylates | |
| acetic acid derivatives and related substances | |
| oxicams | |
| propionic acid derivatives (profens) |
|
| n-arylanthranilic acids (fenamates) | |
| COX-2 inhibitors (coxibs) | |
| other | |
| NSAID combinations | |
| Key: underline indicates initially developed first-in-class compound of specific group; WHO-Essential Medicines; withdrawn drugs; veterinary use. | |
This drug article relating to the musculoskeletal system is a stub. You can help Misplaced Pages by expanding it. |