| |
| Names | |
|---|---|
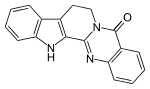
| Preferred IUPAC name 8,13-Hydroindolopyridoquinazolin-5(7H)-one | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.163.752 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C18H13N3O |
| Molar mass | 287.322 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Rutecarpine or rutaecarpine is a COX-2 inhibitor isolated from Tetradium ruticarpum, a tree native to China. It is classified as a non-basic alkaloid.
In contrast to synthetic COX-2 inhibitors like etoricoxib and celecoxib, rutecarpine does not appear to cause negative effects on the cardiovascular system.
Metabolism
Microsome studies suggest that rutaecarpine may be at least a weak inhibitor of CYP1A2, CYP2C9, CYP2C19, CYP2E1, and CYP3A4 enzymes. At the same time, it is believed to be a strong inducer of CYP1A2 and CYP1A1.
Rutecarpine metabolism is complex and proceeds along several routes, primarily involving the addition of a single hydroxyl group by CYP3A4. Six monohydroxylated and four dihydroxylated metabolites have been identified. To a much lesser extent, rutecarpine may be metabolized by CYP2C9 and CYP1A2, according to liver microsome studies.
Supplementation
Rutecarpine has been shown to decrease the overall bioavailability of caffeine in rats by up to 80 percent, likely through induction of enzymes CYP1A2 and CYP2E1.
References
- Moon, T. C.; Murakami, M.; Kudo, I.; Son, K. H.; Kim, H. P.; Kang, S. S.; Chang, H. W. (1999). "A new class of COX-2 inhibitor, rutaecarpine from Evodia rutaecarpa". Inflammation Research. 48 (12): 621–625. doi:10.1007/s000110050512. PMID 10669112. S2CID 19555209.
- Manske, R. H. F. (1950). "Sources of alkaloids and their isolation". In Manske, R. H. F.; Holmes, H. L. (eds.). The Alkaloids: Chemistry and Physiology. Vol. 1. Academic Press. pp. 1–14. doi:10.1016/S1876-0813(08)60184-0. ISBN 978-0-12-469501-6. S2CID 82529003.
- Jia, Sujie; Hu, Changping (2010). "Pharmacological effects of rutaecarpine as a cardiovascular protective agent". Molecules. 15 (3): 1873–1881. doi:10.3390/molecules15031873. PMC 6257227. PMID 20336017. S2CID 21968872.
- Zhang, Fang-Liang; He, Xin; Zhai, Yi-Ran; He, Li-Na; Zhang, Si-Chao; Wang, Li-Li; Yang, Ai-Hong; An, Li-Jun (2 November 2015). "Mechanism-based inhibition of CYPs and RMs-induced hepatoxicity by rutaecarpine". Xenobiotica. 45 (11): 978–989. doi:10.3109/00498254.2015.1038742. PMID 26053557. S2CID 6293291.
- Ueng, Yune-Fang; Jan, Woan-Ching; Lin, Lie-Chwen; Chen, Ta-Liang; Guengerich, F. Peter; Chen, Chieh-Fu (1 March 2002). "The Alkaloid Rutaecarpine Is a Selective Inhibitor of Cytochrome P450 1A in Mouse and Human Liver Microsomes". Drug Metabolism and Disposition. 30 (3): 349–353. doi:10.1124/dmd.30.3.349. PMID 11854157.
- Ueng, Yune-Fang; Wang, Jong-Jing; Lin, Lie-Chwen; Park, Sang Shin; Chen, Chieh-Fu (November 2001). "Induction of cytochrome P450-dependent monooxygenase in mouse liver and kidney by rutaecarpine, an alkaloid of the herbal drug Evodia rutaecarpa". Life Sciences. 70 (2): 207–217. doi:10.1016/S0024-3205(01)01390-X. PMID 11787945.
- Lee, Seung; Son, Jong-Keun; Jeong, Byeong; Jeong, Tae-Cheon; Chang, Hyeon; Lee, Eung-Seok; Jahng, Yurngdong (6 February 2008). "Progress in the Studies on Rutaecarpine". Molecules. 13 (2): 272–300. doi:10.3390/molecules13020272. PMC 6245441. PMID 18305418.
- Estari, Rohit Kumar; Dong, Jin; Chan, William K.; Park, Miki Susanto; Zhou, Zhu (1 December 2021). "Time effect of rutaecarpine on caffeine pharmacokinetics in rats". Biochemistry and Biophysics Reports. 28: 101121. doi:10.1016/j.bbrep.2021.101121. PMC 8429912. PMID 34527815.
- Noh, Keumhan; Seo, Young Min; Lee, Sang Kyu; Bista, Sudeep R.; Kang, Mi Jeong; Jahng, Yurngdong; Kim, Eunyoung; Kang, Wonku; Jeong, Tae Cheon (January 2011). "Effects of rutaecarpine on the metabolism and urinary excretion of caffeine in rats". Archives of Pharmacal Research. 34 (1): 119–125. doi:10.1007/s12272-011-0114-3. PMID 21468923. S2CID 44752343.