 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
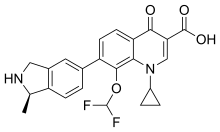
| Formula | C23H20F2N2O4 |
| Molar mass | 426.420 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Garenoxacin (INN) is a quinolone antibiotic for the treatment of Gram-positive and Gram-negative bacterial infections.
Garenoxacin was discovered by Toyama Chemical Co., Ltd. of Tokyo, Japan, and is currently being marketed in Japan under the tradename Geninax. Schering-Plough holds worldwide rights for garenoxacin, except for Japan, South Korea, and China.
On February 13, 2006, Schering-Plough announced that the United States Food and Drug Administration had accepted the New Drug Application (NDA) for garenoxacin, and had been granted a 10-month review. As of 2015, however, it has not been approved in the US.
Schering-Plough later withdrew its application to the United States Food and Drug Administration, FDA, (August 20, 2006) for approval of the antibiotic Garenoxacin.
The European Medicines Agency (EMA) had also been formally notified by Schering-Plough Europe (July 25, 2007) of its decision to withdraw the application for a centralized marketing authorization for garenoxacin as well. Based on the CHMP review of the data regarding safety and efficacy (risk/benefit), the CHMP considered the application for garenoxacin to be unapprovable.
References
- Takagi H, Tanaka K, Tsuda H, Kobayashi H (December 2008). "Clinical studies of garenoxacin". International Journal of Antimicrobial Agents. 32 (6): 468–74. doi:10.1016/j.ijantimicag.2008.06.032. PMID 18790608.
- "Drugs.com, Schering-Plough Reports Garenoxacin NDA Accepted for FDA Review". Retrieved 2008-03-25.
- "Schering-Plough pulls its garenoxacin app". 20 August 2006.
- "Schering-Plough Europe Withdraws Its Marketing Authorisation Application For Garenoxacin Mesylate". MediLexicon International Ltd. 28 July 2007. Archived from the original on 2007-08-08. Retrieved 2009-05-30.
- "Garenoxacin mesylate: Withdrawn application". European Medicines Agency (EMA). 17 September 2018. Retrieved 13 July 2020.
- "Schering-Plough Europe withdraws its marketing authorisation applicationfor Garenoxacin mesylate". European Medicines Agency (EMA) (Press release). Retrieved 13 July 2020.
- "Withdrawal Assessment report for Garenoxacin Mesylate (garenoxacin)" (PDF). European Medicines Agency. 18 October 2007.
| Antibacterials that inhibit nucleic acid (J01E, J01M) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antifolates (inhibit bacterial purine metabolism, thereby inhibiting DNA and RNA synthesis) |
| ||||||||||||||||
| Quinolones (inhibit bacterial topoisomerase and/or DNA gyrase, thereby inhibiting DNA replication) |
| ||||||||||||||||
| Anaerobic DNA inhibitors |
| ||||||||||||||||
| RNA synthesis |
| ||||||||||||||||
| |||||||||||||||||
This systemic antibiotic-related article is a stub. You can help Misplaced Pages by expanding it. |