(Redirected from Rhenium triiodide )
Chemical compound
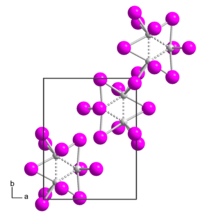
Rhenium(III) iodide is a binary chemical compound of rhenium and iodide with the chemical formula ReI3 .
Synthesis
Rhenium(III) iodide can be synthesized by the decomposition of rhenium(IV) iodide :
2ReI4 → 2ReI3 + I2 Another way to make it is by introduction of ethanol into a mixture of perrhenic acid and hydroiodic acid .
HReO4 + 3HI + 2C2 H5 OH → ReI3 + 4H2 O + 2CH3 CHO Physical properties
Rhenium(III) iodide forms violet-black crystals. It is poorly soluble in water , acetone , ethanol , ether , and dilute acid solutions.
Chemical properties
When heated in vacuum up to 170 °C, the compound decomposes to rhenium(II) iodide, and at 380 °C — to rhenium(I) iodide:
2ReI3 → 2ReI2 + I2
ReI3 → ReI + I2 References
"Rhenium(III) Iodide" . American Elements . Retrieved 6 May 2023."Rhenium(III) iodide" . Alfa Chemistry. Retrieved 6 May 2023.Kemmitt, R. D. W.; Peacock, R. D. (26 January 2016). The Chemistry of Manganese, Technetium and Rhenium: Pergamon Texts in Inorganic Chemistry Elsevier . p. 918. ISBN 978-1-4831-8762-4
Inorganic Syntheses, Volume 7 John Wiley & Sons . 22 September 2009. p. 185. ISBN 978-0-470-13270-8
Rhenium compounds Rhenium(0) Rhenium(I) Rhenium(II)
Rhenium(III)
Rhenium(IV)
Rhenium(V)
Rhenium(VI)
Rhenium(VII)
Salts and covalent derivatives of the iodide ion
Categories :
Text is available under the Creative Commons Attribution-ShareAlike License. Additional terms may apply.
**DISCLAIMER** We are not affiliated with Wikipedia, and Cloudflare.
The information presented on this site is for general informational purposes only and does not constitute medical advice.
You should always have a personal consultation with a healthcare professional before making changes to your diet, medication, or exercise routine.
AI helps with the correspondence in our chat.
We participate in an affiliate program. If you buy something through a link, we may earn a commission 💕
↑