| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.030.070 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | TeBr4 |
| Molar mass | 447.22 g/mol |
| Appearance | yellow-orange crystals |
| Density | 4.3 g/cm, solid |
| Melting point | 388 °C (730 °F; 661 K) |
| Boiling point | decomposes at 420 °C (788 °F; 693 K) |
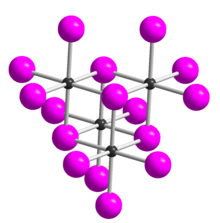
| Structure | |
| Crystal structure | monoclinic |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H301, H314 |
| Precautionary statements | P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 |
| Related compounds | |
| Other anions | Tellurium tetrafluoride Tellurium tetrachloride Tellurium tetraiodide |
| Other cations | Selenium tetrabromide |
| Related compounds | Ditellurium bromide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Tellurium tetrabromide (TeBr4) is an inorganic chemical compound. It has a similar tetrameric structure to TeCl4. It can be made by reacting bromine and tellurium. In the vapour TeBr4 dissociates:
- TeBr4 → TeBr2 + Br2
It is a conductor when molten, dissociating into the ions TeBr3 and Br. When dissolved in benzene and toluene, TeBr4 is present as the unionized tetramer Te4Br16. In solvents with donor properties such as acetonitrile, CH3CN ionic complexes are formed which make the solution conducting:
- TeBr4 + 2CH3CN → (CH3CN)2TeBr3 + Br
References
- Thermochemical Data of Elements and Compounds, M. Binnewies, E. Milke, Wiley-VCH, 2002, ISBN 3-527-30524-6
- "C&L Inventory". echa.europa.eu.
- ^ Inorganic Chemistry,Egon Wiberg, Arnold Frederick Holleman Elsevier 2001 ISBN 0-12-352651-5
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
| Tellurium compounds | |
|---|---|
| Bromine compounds | |
|---|---|
| Br(−I) | |
| Br(−I,I) | |
| Br(I) | |
| Br(II) | |
| Br(I,V) | |
| Br(III) | |
| Br(IV) | |
| Br(V) | |
| Br(VII) | |
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |