
| |
| Names | |
|---|---|
| IUPAC name Tungsten(VI) dichloride dioxide | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.033.496 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | WO2Cl2 |
| Molar mass | 286.74 g·mol |
| Appearance | Yellow-red crystals |
| Density | 4.67 g/cm |
| Melting point | 265 °C (509 °F; 538 K) |
| Boiling point | Sublimes at > 350 °C in vacuum |
| Solubility in water | decomposes |
| Solubility | slightly soluble in ethanol |
| Structure | |
| Crystal structure | orthorhombic |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Tungsten dichloride dioxide, or Tungstyl chloride is the chemical compound with the formula WO2Cl2. It is a yellow-colored solid. It is used as a precursor to other tungsten compounds. Like other tungsten halides, WO2Cl2 is sensitive to moisture, undergoing hydrolysis.
Preparation
WO2Cl2 is prepared by ligand redistribution reaction from tungsten trioxide and tungsten hexachloride:
- 2 WO3 + WCl6 → 3 WO2Cl2
Using a two-zone tube furnace, a vacuum-sealed tube containing these solids is heated to 350 °C. The yellow product sublimes to the cooler end of the reaction tube. No redox occurs in this process. An alternative route highlights the oxophilicity of tungsten:
- WCl6 + 2 ((CH3)3Si)2O → 3 WO2Cl2 + 4 (CH3)3SiCl
This reaction, like the preceding one, proceeds via the intermediacy of WOCl4.
Structure
Gaseous tungsten dichloride dioxide is a monomer. Solid tungsten dichloride dioxide is a polymer consisting of distorted octahedral W centres. The polymer is characterized by two short W-O distances, typical for a multiple W-O bond, and two long W-O distances more typical of a single or dative W-O bond.
Related oxy halides
Tungsten forms a number of oxyhalides including WOCl4, WOCl3, WOCl2. The corresponding bromides (WOBr4, WOBr3, WOBr2) are also known as is WO2I2.
Reactions
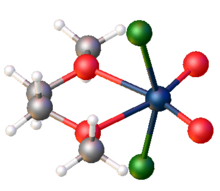
WO2Cl2 is a Lewis acid, forming soluble adducts of the type WO2Cl2L2, where L is a donor ligand such as bipyridine and dimethoxyethane. Such complexes often cannot be prepared by depolymerization of the inorganic solid, but are generated in situ from WOCl4.
References
- "C&L Inventory". echa.europa.eu. Retrieved 12 December 2021.
- Tillack, J. (1973). "Tungsten Oxyhalides". Inorganic Syntheses. Vol. 14. pp. 109–122. doi:10.1002/9780470132456.ch22. ISBN 9780470132456.
{{cite book}}:|journal=ignored (help) - Gibson, V. C.; Kee, T. P.; Shaw, A. (1988). "New, improved synthesis of the group 6 oxyhalides, W(O)Cl4, W(O)2Cl2 and Mo(O)2Cl2". Polyhedron. 7 (7): 579–80. doi:10.1016/S0277-5387(00)86336-6.
- Ward, Brian G.; Stafford, Fred E. (1968). "Synthesis and Structure of Four- and Five-Coordinated Gaseous Oxohalides of Molybdenum(VI) and Tungsten(VI)". Inorganic Chemistry. 7 (12): 2569–2573. doi:10.1021/ic50070a020.
- Jarchow, O.; Schröder, F.; Schulz, H. "Kristallstruktur und Polytypie von WO2Cl2" Zeitschrift für anorganische und allgemeine Chemie 1968, vol. 363, p. 345ff. doi:10.1002/zaac.19683630108
- Holleman, A. F.; Wiberg, E. Inorganic Chemistry Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- K. Dreisch, C. Andersson, C. Stalhandske "Synthesis and structure of dimethoxyethane-dichlorodioxo-tungsten(VI)—a highly soluble derivative of tungsten dioxodichloride" Polyhedron 1991, volume 10, p. 2417. doi:10.1016/S0277-5387(00)86203-8
| Tungsten compounds | |||||
|---|---|---|---|---|---|
| Tungsten(0) | |||||
| Tungsten(II) | |||||
| Tungsten(III) | |||||
| Tungsten(IV) | |||||
| Tungsten(V) | |||||
| Tungsten(VI) |
| ||||