| Revision as of 16:17, 20 October 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (changes to verified fields - updated 'ChEBI_Ref', 'CAS_number_Ref') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Latest revision as of 11:41, 21 October 2024 edit undoJWBE (talk | contribs)Extended confirmed users10,127 edits removed Category:Cyclopropanes; added Category:Cyclopropyl compounds using HotCat | ||
| (21 intermediate revisions by 20 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Drugbox | {{Drugbox | ||
| | Verifiedfields = changed | | Verifiedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 462265582 | ||
| | IUPAC_name = 1-Cyclopropyl-7--5,6,8-trifluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid | | IUPAC_name = 1-Cyclopropyl-7--5,6,8-trifluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid | ||
| | image = orbifloxacin.png | | image = orbifloxacin.png | ||
| <!--Clinical data--> | <!--Clinical data--> | ||
| | tradename = |
| tradename = | ||
| | Drugs.com = {{drugs.com|international|orbifloxacin}} | | Drugs.com = {{drugs.com|international|orbifloxacin}} | ||
| | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| | pregnancy_US = <!-- A / B / C / D / X --> | | pregnancy_US = <!-- A / B / C / D / X --> | ||
| | pregnancy_category = |
| pregnancy_category = | ||
| | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | ||
| | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | ||
| Line 19: | Line 20: | ||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| | bioavailability = |
| bioavailability = | ||
| | protein_bound = |
| protein_bound = | ||
| | metabolism = |
| metabolism = | ||
| | elimination_half-life = |
| elimination_half-life = | ||
| | excretion = |
| excretion = | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | CAS_number_Ref = {{cascite| |
| CAS_number_Ref = {{cascite|changed|??}} | ||
| | CAS_number = 113617-63-3 | | CAS_number = 113617-63-3 | ||
| | ATCvet = yes | | ATCvet = yes | ||
| Line 33: | Line 34: | ||
| | PubChem = 60605 | | PubChem = 60605 | ||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | DrugBank = |
| DrugBank = | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 54631 | | ChemSpiderID = 54631 | ||
| | UNII_Ref = {{fdacite| |
| UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = 660932TPY6 | | UNII = 660932TPY6 | ||
| | KEGG_Ref = {{keggcite|correct|kegg}} | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| Line 44: | Line 45: | ||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=19 | H=20 | F=3 | N=3 | O=3 |
| C=19 | H=20 | F=3 | N=3 | O=3 | ||
| ⚫ | | smiles = O=C(O)C1=CN(C2CC2)c3c(C1=O)c(F)c(F)c(c3F)N4C(C)N(C)C4 | ||
| | molecular_weight = 395.37 g/mol | |||
| ⚫ | | smiles = |
||
| | InChI = 1/C19H20F3N3O3/c1-8-5-24(6-9(2)23-8)17-14(21)13(20)12-16(15(17)22)25(10-3-4-10)7-11(18(12)26)19(27)28/h7-10,23H,3-6H2,1-2H3,(H,27,28)/t8-,9+ | |||
| | InChIKey = QIPQASLPWJVQMH-DTORHVGOBR | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C19H20F3N3O3/c1-8-5-24(6-9(2)23-8)17-14(21)13(20)12-16(15(17)22)25(10-3-4-10)7-11(18(12)26)19(27)28/h7-10,23H,3-6H2,1-2H3,(H,27,28)/t8-,9+ | | StdInChI = 1S/C19H20F3N3O3/c1-8-5-24(6-9(2)23-8)17-14(21)13(20)12-16(15(17)22)25(10-3-4-10)7-11(18(12)26)19(27)28/h7-10,23H,3-6H2,1-2H3,(H,27,28)/t8-,9+ | ||
| Line 55: | Line 53: | ||
| }} | }} | ||
| '''Orbifloxacin''' (brand name '''Orbax''') is a ] ] which is approved for use in dogs,<ref> |
'''Orbifloxacin''' (brand name '''Orbax''') is a ] ] which is approved for use in dogs,<ref>{{cite web | url = http://dil.vetmed.vt.edu/Display/NadaBrowse.cfm?NadaString=141-081 | title = Product Abstract - NADA 141-081 | access-date = 21 June 2008 | publisher = American College of Veterinary Clinical Pharmacology | url-status = }}{{deadlink|date=September 2023}}</ref><ref>{{cite book | vauthors = Papich MG | chapter = Orbifloxacin | chapter-url = https://books.google.com/books?id=MVcBEAAAQBAJ&pg=PA674 | page = 674 | title=Papich Handbook of Veterinary Drugs |date= October 2020 |location=St. Louis, Missouri | publisher = Elsevier Health Sciences |isbn=978-0-323-70958-3 |edition=Fifth}}</ref> marketed by ]. | ||
| ==See also== | ==See also== | ||
| ] | |||
| *] | |||
| * ] | |||
| *] | |||
| ==References== | ==References== | ||
| Line 67: | Line 65: | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
Latest revision as of 11:41, 21 October 2024
Chemical compound Pharmaceutical compound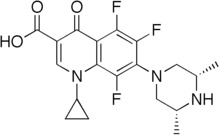 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.166.510 |
| Chemical and physical data | |
| Formula | C19H20F3N3O3 |
| Molar mass | 395.382 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Orbifloxacin (brand name Orbax) is a fluoroquinolone antibiotic which is approved for use in dogs, marketed by Schering-Plough Animal Health.
See also

References
- "Product Abstract - NADA 141-081". American College of Veterinary Clinical Pharmacology. Retrieved 21 June 2008.
- Papich MG (October 2020). "Orbifloxacin". Papich Handbook of Veterinary Drugs (Fifth ed.). St. Louis, Missouri: Elsevier Health Sciences. p. 674. ISBN 978-0-323-70958-3.
| Antibacterials that inhibit nucleic acid (J01E, J01M) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antifolates (inhibit bacterial purine metabolism, thereby inhibiting DNA and RNA synthesis) |
| ||||||||||||||||
| Quinolones (inhibit bacterial topoisomerase and/or DNA gyrase, thereby inhibiting DNA replication) |
| ||||||||||||||||
| Anaerobic DNA inhibitors |
| ||||||||||||||||
| RNA synthesis |
| ||||||||||||||||
| |||||||||||||||||
This systemic antibiotic-related article is a stub. You can help Misplaced Pages by expanding it. |
This veterinary medicine–related article is a stub. You can help Misplaced Pages by expanding it. |