| Revision as of 22:51, 8 December 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (changes to watched fields - added verified revid - updated 'DrugBank_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Latest revision as of 22:02, 5 January 2025 edit undoJhgarrison (talk | contribs)12 editsm →Occurrence as natural mineral: spelling correction "nitrite" -> "natrite" | ||
| (708 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Refimprove section|date=May 2010}} | |||
| {{Distinguish|text= ] (baking soda), a similar compound}} | |||
| {{wikify|date=September 2011}} | |||
| {{chembox | {{chembox | ||
| | |
| Verifiedfields = changed | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 464362057 | |||
| | verifiedrevid = 464362057 | |||
| | Name = Sodium carbonate | |||
| | |
| Name = Sodium carbonate | ||
| | ImageFile = Sodium carbonate.svg | |||
| | ImageSize = 180px | |||
| | ImageSize = 120px | |||
| | ImageName = Structural formula of sodium carbonate | |||
| | ImageName = Skeletal formula of sodium carbonate | |||
| | ImageFile1 = Uhličitan sodný.JPG | |||
| | ImageFile1 = Uhličitan sodný.JPG | |||
| | ImageName1 = Sodium carbonate | |||
| | |
| ImageFile2 = Sodium-carbonate-xtal-3D-SF-C.png | ||
| | |
| ImageName1 = Sample of sodium carbonate | ||
| | IUPACName = Sodium carbonate | |||
| | OtherNames = Soda ash<br/>Washing soda<br/>Soda crystals | |||
| | OtherNames = Soda ash, washing soda, soda crystals, sodium trioxocarbonate | |||
| | Section1 = {{Chembox Identifiers | |||
| | Section1 = {{Chembox Identifiers | |||
| | CASNo = 497-19-8 | |||
| | |
|CASNo_Ref = {{cascite|correct|CAS}} | ||
| |CASNo = 497-19-8 | |||
| | CASOther = <br>5968-11-6 (monohydrate)<br>6132-02-1 (decahydrate) | |||
| |CASNo_Comment = (anhydrous) | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| |CASNo1_Ref = {{cascite|correct|CAS}} | |||
| | ChEMBL = 186314 | |||
| |CASNo1 = 5968-11-6 | |||
| | PubChem = 10340 | |||
| |CASNo1_Comment = (monohydrate) | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| |CASNo2_Ref = {{cascite|correct|CAS}} | |||
| | ChemSpiderID = 9916 | |||
| |CASNo2 = 6132-02-1 | |||
| | RTECS = VZ4050000 | |||
| |CASNo2_Comment = (decahydrate) | |||
| | EINECS = 207-838-8| UNII_Ref = {{fdacite|correct|FDA}} | |||
| |ChEMBL = 186314 | |||
| | UNII = 45P3261C7T | |||
| |ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | InChI = 1/CH2O3.2Na/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2 | |||
| |PubChem = 10340 | |||
| | InChIKey = CDBYLPFSWZWCQE-NUQVWONBAP | |||
| |ChemSpiderID = 9916 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| |ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChEBI = 29377 | |||
| |RTECS = VZ4050000 | |||
| | SMILES = ..C()=O | |||
| |EC_number = 207-838-8 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| |UNII = 45P3261C7T | |||
| | StdInChI = 1S/CH2O3.2Na/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2 | |||
| | |
|UNII_Ref = {{fdacite|correct|FDA}} | ||
| |UNII1_Ref = {{fdacite|correct|FDA}} | |||
| | StdInChIKey = CDBYLPFSWZWCQE-UHFFFAOYSA-L}} | |||
| |UNII1 = 2A1Q1Q3557 | |||
| | Section2 = {{Chembox Properties | |||
| |UNII1_Comment = (monohydrate) | |||
| | Formula = Na<sub>2</sub>CO<sub>3</sub> | |||
| |UNII2_Ref = {{fdacite|correct|FDA}} | |||
| | MolarMass = 105.9784 g/mol (anhydrous) <br> 124.00 g/mol (monohydrate) <br> 286.14 g/mol (decahydrate) | |||
| |UNII2 = LS505BG22I | |||
| | Appearance = White solid, ] | |||
| |UNII2_Comment = (decahydrate) | |||
| | Odor = Odorless | |||
| |InChI = 1/NaHCO3.2Na/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2 | |||
| | Density = 2.54 g/cm<sup>3</sup> (anhydrous) <br> 2.25 g/cm<sup>3</sup> (monohydrate) <br> 1.46 g/cm<sup>3</sup> (decahydrate) | |||
| |InChIKey = CDBYLPFSWZWCQE-NUQVWONBAP | |||
| | Solubility = 70 g/L (0 °C)<br/>216 g/L (20 °C)<ref name=UNEP>{{cite web|publisher = UNEP Publications|url = http://www.chem.unep.ch/irptc/sids/oecdsids/Naco.pdf|title = Sodium Carbonate}}</ref><br/>450 g/L (100 °C)<ref name="ndctz.com">{{dead link|date=June 2011}}</ref> | |||
| |ChEBI = 29377 | |||
| | HeatofSolution = 24.7 kJ/mol at 100 mol H<sub>2</sub>O<ref name="ndctz.com"/> | |||
| |ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | SolubleOther = insoluble in ] | |||
| |SMILES = ..C()=O | |||
| | MeltingPt = 851 °C (anhydrous)<ref name="UNEP"/> <br> 100 °C (decomp, monohydrate) <br> 34 °C (decomp, decahydrate) | |||
| |StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | BoilingPt = 1633 °C (anhydrous) | |||
| |StdInChI = 1S/CH2O3.2Na/c2-1(3)4;;/h(H2,2,3,4);;/q;2*+1/p-2 | |||
| | pKb = 4.67 | |||
| |StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | RefractIndex = 1.485 (anhydrous) <br> 1.420 (monohydrate) | |||
| |StdInChIKey = CDBYLPFSWZWCQE-UHFFFAOYSA-L | |||
| }} | |||
| }} | |||
| | Section3 = {{Chembox Structure | |||
| | Section2 = {{Chembox Properties | |||
| | Coordination = trigonal planar | |||
| |Formula = Na<sub>2</sub>CO<sub>3</sub> | |||
| | Structure = triclinic (anhydrous) <br> orthorhombic (monohydrate) | |||
| |MolarMass = 105.9888{{nbsp}}g/mol (anhydrous)<br />286.1416{{nbsp}}g/mol (decahydrate) | |||
| }} | |||
| |Appearance = White solid, ] | |||
| | Section7 = {{Chembox Hazards | |||
| |Odor = Odorless | |||
| | ExternalMSDS = | |||
| |Density = {{ubl | |||
| | EUIndex = 011-005-00-2 | |||
| | 2.54{{nbsp}}g/cm<sup>3</sup> (25 °C, anhydrous) | |||
| | EUClass = Irritant ('''Xi''') | |||
| | 1.92{{nbsp}}g/cm<sup>3</sup> (856 °C) | |||
| | NFPA-H = 1 | |||
| | 2.25{{nbsp}}g/cm<sup>3</sup> (monohydrate)<ref name=cod /> | |||
| | NFPA-F = 0 | |||
| | 1.51{{nbsp}}g/cm<sup>3</sup> (heptahydrate) | |||
| | NFPA-R = 1 | |||
| | 1.46{{nbsp}}g/cm<sup>3</sup> (decahydrate)<ref name=crc /> | |||
| | RPhrases = {{R36}} | |||
| }} | |||
| | SPhrases = {{S2}}, {{S22}}, {{S26}} | |||
| | Solubility = Anhydrous, g/100{{nnbsp}}mL:{{ubl | |||
| | FlashPt = Non-flammable | |||
| | 7 (0 °C) | |||
| }} | |||
| | 16.4 (15 °C) | |||
| | Section8 = {{Chembox Related | |||
| | 34.07 (27.8 °C) | |||
| | OtherAnions = ] | |||
| | 48.69 (34.8 °C) | |||
| | OtherCations = ]<br/>]<br/>]<br/>] | |||
| | 48.1 (41.9 °C) | |||
| | OtherCpds = ]<br/>]<br/>] | |||
| | 45.62 (60 °C) | |||
| }} | |||
| | 43.6 (100 °C)<ref name=sioc>{{cite book|last1=Seidell |first1=Atherton |last2=Linke |first2=William F. |year=1919 |title=Solubilities of Inorganic and Organic Compounds |url=https://archive.org/details/solubilitiesino01seidgoog |publisher=D. Van Nostrand Company |place=] |edition=2nd |page=}}</ref> | |||
| }} | |||
| |SolubleOther = Soluble in aq. ]s,<ref name=sioc /> ]<br /> Slightly soluble in aq. ]<br /> Insoluble in ], ], alkyl ]s, alcohol, ], liquid ]<ref name=doc00>{{cite book|title = A Dictionary of Chemical Solubilities: Inorganic|url = https://archive.org/details/in.ernet.dli.2015.163725|edition = 2nd|first1 = Arthur Messinger|last1 = Comey|first2 = Dorothy A.|last2 = Hahn|place = New York|publisher = The MacMillan Company|date = February 1921|pages = 208–209}}</ref> | |||
| |Solubility1 = 98.3{{nbsp}}g/100{{nnbsp}}g (155 °C)<ref name=doc00 /> | |||
| |Solvent1 = glycerine | |||
| |Solubility2 = 3.46{{nbsp}}g/100{{nnbsp}}g (20 °C)<ref name=chemister /> | |||
| |Solvent2 = ethanediol | |||
| |Solubility3 = 0.5{{nbsp}}g/kg<ref name=chemister /> | |||
| |Solvent3 = dimethylformamide | |||
| |MeltingPtC = 851 | |||
| |MeltingPt_notes = (Anhydrous)<br /> {{convert|100|C|F K}}<br /> decomposes (monohydrate)<br /> {{convert|33.5|C|F K}}<br /> decomposes (heptahydrate)<br /> {{convert|34|C|F K}}<br /> (decahydrate)<ref name=crc>{{CRC90}}</ref><ref name=pphoic>{{cite book|last = Pradyot|first = Patnaik|year = 2003|title = Handbook of Inorganic Chemicals|publisher = McGraw-Hill |isbn = 978-0-07-049439-8|page = 861}}</ref> | |||
| |pKa = 10.33 | |||
| |RefractIndex = 1.485 (anhydrous)<br /> 1.420 (monohydrate)<ref name=pphoic /><br /> 1.405 (decahydrate) | |||
| |MagSus = −4.1·10<sup>−5</sup> cm<sup>3</sup>/mol<ref name=crc /> | |||
| |Viscosity = 3.4 cP (887 °C)<ref name=chemister /> | |||
| }} | |||
| | Section3 = {{Chembox Structure | |||
| | CrystalStruct = ] (γ-form, β-form, δ-form, anhydrous)<ref name=scr>{{cite journal|title = Sodium carbonate revisited|first1 = Michal|last1 = Dusek|first2 = Gervais|last2 = Chapuis|first3 = Mathias|last3 = Meyer|first4 = Vaclav|last4 = Petricek|journal = ]|url = http://infoscience.epfl.ch/record/82110/files/publ_03_dusek_a.pdf|issn = 0108-7681|year = 2003|volume = 59|issue = 3|access-date = 2014-07-25|pages = 337–352|doi = 10.1107/S0108768103009017|pmid = 12761404| bibcode=2003AcCrB..59..337D }}</ref><br /> ] (monohydrate, heptahydrate)<ref name=cod>{{cite journal|title = Crystal Structure of Sodium Carbonate Monohydrate, Na<sub>2</sub>CO<sub>3</sub>. H<sub>2</sub>O|first = J. P.|last = Harper|journal = Zeitschrift für Kristallographie - Crystalline Materials|url = http://www.crystallography.net/1011295.html|issn = 2196-7105|access-date = 2014-07-25|pages = 266–273|year = 1936|volume = 95|issue = 1|doi = 10.1524/zkri.1936.95.1.266|editor-last1 = Antipov|editor-first1 = Evgeny|editor-last2 = Bismayer|editor-first2 = Ulrich|editor-last3 = Huppertz|editor-first3 = Hubert|editor-last4 = Petrícek|editor-first4 = Václav|editor-last5 = Pöttgen|editor-first5 = Rainer|editor-last6 = Schmahl|editor-first6 = Wolfgang|editor-last7 = Tiekink|editor-first7 = E. R. T. |editor-last8 = Zou|editor-first8 = Xiaodong}}</ref><ref name=7h2o>{{cite journal|title = Sodium Carbonate Heptahydrate|first1 = C.|last1 = Betzel|first2 = W.|last2 = Saenger|first3 = D.|last3 = Loewus|journal = Acta Crystallographica Section B|pages = 2802–2804|year = 1982|volume = 38|issue = 11|doi = 10.1107/S0567740882009996| bibcode=1982AcCrB..38.2802B }}</ref> | |||
| |SpaceGroup = C2/m, No. 12 (γ-form, anhydrous, 170 K)<br /> C2/m, No. 12 (β-form, anhydrous, 628 K)<br /> P2<sub>1</sub>/n, No. 14 (δ-form, anhydrous, 110 K)<ref name=scr /><br /> Pca2<sub>1</sub>, No. 29 (monohydrate)<ref name=cod /><br /> Pbca, No. 61 (heptahydrate)<ref name=7h2o /> | |||
| |PointGroup = 2/m (γ-form, β-form, δ-form, anhydrous)<ref name=scr /><br /> mm2 (monohydrate)<ref name=cod /><br /> 2/m 2/m 2/m (heptahydrate)<ref name=7h2o /> | |||
| |LattConst_a = 8.920(7) Å | |||
| |LattConst_b = 5.245(5) Å | |||
| |LattConst_c = 6.050(5) Å (γ-form, anhydrous, 295 K)<ref name=scr /> | |||
| |LattConst_beta = 101.35(8) | |||
| |Coordination = Octahedral (Na<sup>+</sup>, anhydrous) | |||
| }} | |||
| | Section4 = {{Chembox Thermochemistry | |||
| |DeltaHf = −1130.7{{nbsp}}kJ/mol<ref name=crc /><ref name=chemister>{{cite web|last = Anatolievich|first = Kiper Ruslan|website =chemister.ru|url = http://chemister.ru/Database/properties-en.php?dbid=1&id=66|title = sodium carbonate|access-date = 2014-07-25}}</ref> | |||
| |Entropy = 135{{nbsp}}J/mol·K<ref name=crc /> | |||
| |DeltaGf = −1044.4{{nbsp}}kJ/mol<ref name=crc /> | |||
| |HeatCapacity = 112.3{{nbsp}}J/mol·K<ref name=crc /> | |||
| }} | |||
| | Section5 = {{Chembox Hazards | |||
| |MainHazards = Irritant | |||
| |ExternalSDS = | |||
| |GHSPictograms = {{GHS07}}<ref name="sigma">{{Sigma-Aldrich|id=451614|name=Sodium carbonate|accessdate=2014-05-06}}</ref> | |||
| |GHSSignalWord = Warning | |||
| |HPhrases = {{H-phrases|319|313+333}}<ref name="sigma" /> | |||
| |PPhrases = {{P-phrases|305+351+338}}<ref name="sigma" /> | |||
| |NFPA-H = 2 | |||
| |NFPA-F = 0 | |||
| |NFPA-R = 0 | |||
| |NFPA_ref = <ref name=css>{{cite web|title = Material Safety Data Sheet – Sodium Carbonate, Anhydrous|url = http://www.conservationsupportsystems.com/system/assets/msds/sodium_carbonate_msds.pdf|website =conservationsupportsystems.com|publisher = ConservationSupportSystems|access-date = 2014-07-25}}</ref> | |||
| |LD50 = 4090 mg/kg (rat, oral)<ref>{{cite web|url=https://chem.nlm.nih.gov/chemidplus/rn/497-19-8|title=ChemIDplus - 497-19-8 - CDBYLPFSWZWCQE-UHFFFAOYSA-L - Sodium carbonate - Similar structures search, synonyms, formulas, resource links, and other chemical information|first=Michael|last=Chambers}}</ref> | |||
| }} | |||
| | Section6 = {{Chembox Related | |||
| |OtherAnions = ] | |||
| |OtherCations = ]<br /> ]<br />]<br /> ] | |||
| |OtherCompounds = ]<br /> ] | |||
| }} | |||
| }} | }} | ||
| '''Sodium carbonate''' (also known as '''washing soda''' or '''soda ash'''), Na<sub>2</sub>CO<sub>3</sub> is a ] ] of ]. It most commonly occurs as a ]line heptahydrate, which readily ] to form a white powder, the monohydrate. Sodium carbonate is domestically well known for its everyday use as a ]. It can be extracted from the ashes of many plants. It is synthetically produced in large quantities from salt and ] in a process known as the ]. | |||
| '''Sodium carbonate''' (also known as '''washing soda''', '''soda ash''' and '''soda crystals''') is the ] with the formula {{chem2|Na2CO3|}} and its various ]s. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce ]), sodium carbonate became known as "soda ash".<ref>{{cite web|url=https://www.usgs.gov/centers/national-minerals-information-center/soda-ash-statistics-and-information|title=Soda Ash Statistics and Information|publisher=United States Geographical Survey|access-date=2024-03-03}}</ref> It is produced in large quantities from ] and ] by the ], as well as by carbonating sodium hydroxide which is made using the ]. | |||
| ==Uses== | |||
| {{refimprove section|date=September 2011}} | |||
| *The manufacture of ] is one of the most important uses of sodium carbonate. When combined with ] and ] and heated to high temperatures, then cooled rapidly, glass is produced. This type of glass is known as soda lime glass. | |||
| ==Hydrates== | |||
| *Sodium carbonate is also used as a relatively strong base in various settings. For example, sodium carbonate is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic developing agents. | |||
| Sodium carbonate is obtained as three ]s and as the anhydrous salt: | |||
| *It is a common additive in municipal pools used to neutralize the acidic effects of chlorine and raise pH. | |||
| * sodium carbonate decahydrate (]), Na<sub>2</sub>CO<sub>3</sub>·10H<sub>2</sub>O, which readily ] to form the monohydrate. | |||
| * In cooking, it is sometimes used in place of sodium hydroxide for ]ing, especially with ] and lye rolls. These dishes are treated with a solution of an alkaline substance in order to change the pH of the surface of the food and thus improve browning. | |||
| * sodium carbonate heptahydrate (not known in mineral form), Na<sub>2</sub>CO<sub>3</sub>·7H<sub>2</sub>O. | |||
| * sodium carbonate monohydrate (]), Na<sub>2</sub>CO<sub>3</sub>·H<sub>2</sub>O. Also known as '''crystal carbonate'''. | |||
| * anhydrous sodium carbonate (natrite), also known as calcined soda, is formed by heating the hydrates. It is also formed when sodium hydrogencarbonate is heated (calcined) e.g. in the final step of the ]. | |||
| The decahydrate is formed from water solutions crystallizing in the temperature range −2.1 to +32.0 °C, the heptahydrate in the narrow range 32.0 to 35.4 °C and above this temperature the monohydrate forms.<ref>{{cite journal|title=On the transition temperatures of the transition temperatures of the hydrates of sodium carbonate as fix points in thermometry |journal=Journal of the American Chemical Society |volume=36 |issue=3 |pages=485–490 |author= T.W.Richards and A.H. Fiske|doi=10.1021/ja02180a003 |year=1914 |url=https://zenodo.org/record/1428987}}</ref> In dry air the decahydrate and heptahydrate lose water to give the monohydrate. Other hydrates have been reported, e.g. with 2.5 units of water per sodium carbonate unit ("Penta hemihydrate").<ref>{{cite web |url=http://www.minsocam.org/ammin/am15/am15_69.pdf |author=A. Pabst |title=On the hydrates of sodium carbonate }}</ref> | |||
| ===Washing soda=== | |||
| *In ], sodium carbonate added to boiling water will remove flesh from the skull or bones of trophies to create the "European skull mount" or for educational display in biological and historical studies. | |||
| Sodium carbonate decahydrate (Na<sub>2</sub>CO<sub>3</sub>·10H<sub>2</sub>O), also known as washing soda, is the most common hydrate of sodium carbonate containing 10 molecules of ]. Soda ash is dissolved in water and crystallized to get washing soda. | |||
| <chem display="block">Na2CO3 + 10H2O -> Na2CO3.10H2O</chem> | |||
| *In chemistry, it is often used as an electrolyte. This is because electrolytes are usually salt-based, and sodium carbonate acts as a very good conductor in the process of electrolysis. In addition, unlike chloride ions, which form chlorine gas, carbonate ions are not corrosive to the anodes. It is also used as a primary standard for acid-base ]s because it is solid and air-stable, making it easy to weigh accurately. | |||
| It is one of the few metal ]s that is soluble in water. | |||
| ===Domestic use=== | |||
| ==Applications== | |||
| *In domestic use, it is used as a water softener during laundry. It competes with the ions magnesium and calcium in hard water and prevents them from bonding with the detergent being used. Without using washing soda, additional detergent is needed to soak up the magnesium and calcium ions. Called '''washing soda''', '''soda crystals''', or '''sal soda''' in the detergent section of stores, it effectively removes oil, grease, and alcohol stains. Sodium carbonate is also used as a descaling agent in boilers such as those found in coffee pots, ]s, etc. | |||
| Some common applications of sodium carbonate include: | |||
| * As a cleansing agent for domestic purposes like washing clothes. Sodium carbonate is a component of many dry soap powders. It has ] properties through the process of ], which converts fats and grease to water-soluble ]s (specifically, soaps).<ref name=Ullmann/> | |||
| * It is used for lowering the ]<ref name="Cornell" /> (see {{section link|#Water softening}}). | |||
| * It is used in the manufacture of ],<ref name="Himmel">{{Cite book |last=Himmelblau |first=David M. |title=Basic principles and calculations in chemical engineering |last2=Riggs |first2=James B. |date=2022 |publisher=Pearson |isbn=978-0-13-732717-1 |edition=Ninth |series=International series in the physical and chemical engineering sciences |location=Boston}}</ref> ],<ref name="Himmel" /> and ] (see {{section link|#Glass manufacture}}). | |||
| * It is used in the manufacture of sodium compounds like ] (sodium borate). | |||
| ===Glass manufacture=== | |||
| *In dyeing with fiber-reactive dyes, sodium carbonate (often under a name such as soda ash fixative or soda ash activator) is used to ensure proper chemical bonding of the dye with cellulose (plant) fibers, typically before dyeing (for tie dyes), mixed with the dye (for dye painting), or after dyeing (for immersion dyeing). | |||
| Sodium carbonate serves as a ] for ] (SiO<sub>2</sub>, melting point 1,713 °C), lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some ] is added to the melt mixture to make the glass insoluble. Bottle and window glass ("]" with transition temperature ~570 °C) is made by melting such mixtures of sodium carbonate, calcium carbonate, and silica sand (] (SiO<sub>2</sub>)). When these materials are heated, the carbonates release carbon dioxide. In this way, sodium carbonate is a source of sodium oxide. Soda–lime glass has been the most common form of glass for centuries. It is also a key input for tableware glass manufacturing.<ref name=Ullmann/> | |||
| ===Water softening=== | |||
| *Sodium carbonate is a powerful electrolyte, and is therefore used to speed up the decomposition of water in electrolysis. | |||
| {{See also|Hard water|Water softening}} | |||
| Hard water usually contains calcium or magnesium ions. Sodium carbonate is used for removing these ions and replacing them with sodium ions.<ref name="Cornell">{{cite web |url=https://www.ccmr.cornell.edu/wp-content/uploads/sites/2/2015/11/Water-Hardness-Reading.pdf |title=Water Hardness Reading |website=Cornell Center for Materials Research}}</ref> | |||
| Sodium carbonate is a water-soluble source of carbonate. The calcium and magnesium ions form insoluble solid precipitates upon treatment with ] ions: | |||
| {{block indent|{{chem2|Ca(2+) + CO3(2-) -> CaCO3 (s)}}}} | |||
| The water is softened because it no longer contains dissolved calcium ions and magnesium ions.<ref name="Cornell" /> | |||
| ===Food additive and cooking=== | |||
| Sodium carbonate has several uses in cuisine, largely because it is a stronger base than baking soda (]) but weaker than ] (which may refer to ] or, less commonly, ]). Alkalinity affects ] production in kneaded doughs, and also improves browning by reducing the temperature at which the ] occurs. To take advantage of the former effect, sodium carbonate is therefore one of the components of {{nihongo3||かん水|kansui}}, a solution of alkaline salts used to give ] ] noodles their characteristic flavour and chewy texture; a similar solution is used in ] to make ], for similar reasons. ] bakers similarly use sodium carbonate as a substitute for lye-water to give ]s their characteristic texture and improve browning. In ] (and Central European cuisine more broadly), breads such as ]s and ]s traditionally treated with lye to improve browning can be treated instead with sodium carbonate; sodium carbonate does not produce quite as strong a browning as lye, but is much safer and easier to work with.<ref name="McGee">{{cite news |last1=McGee |first1=Harold |author-link=Harold McGee |title=For Old-Fashioned Flavor, Bake the Baking Soda |url=https://www.nytimes.com/2010/09/15/dining/15curious.html |access-date=25 April 2019 |work=] |date=24 September 2010}}</ref> | |||
| Sodium carbonate is used in the production of ] powder. The cooling and fizzing sensation results from the endothermic reaction between sodium carbonate and a weak acid, commonly ], releasing carbon dioxide gas, which occurs when the sherbet is moistened by saliva. | |||
| Sodium carbonate also finds use in the ] as a ] (] E500) as an acidity regulator, ], ], and stabilizer. It is also used in the production of {{lang|no|]}} to stabilize the pH of the final product. | |||
| While it is less likely to cause chemical burns than lye, care must still be taken when working with sodium carbonate in the kitchen, as it is corrosive to aluminum cookware, utensils, and foil.{{tone inline|date=November 2024}}<ref>{{cite web |title=Sodium Carbonate |url=https://www.corrosionpedia.com/definition/2782/sodium-carbonate |website=corrosionpedia |publisher=Janalta Interactive |access-date=9 November 2020}}</ref>{{unreliable source|date=November 2024}} | |||
| ===Other applications=== | ===Other applications=== | ||
| Sodium carbonate is also used as a relatively strong ] in various fields. As a common alkali, it is preferred in many chemical processes because it is cheaper than ] and far safer to handle. Its mildness especially recommends its use in domestic applications. | |||
| *Sodium carbonate is a food additive (E500) used as an acidity regulator, anti-caking agent, raising agent, and stabilizer. It is one of the components of ''kansui'', a solution of alkaline salts used to give ] noodles their characteristic flavor and texture. | |||
| For example, it is used as a ] regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic ] agents. It is also a common additive in ]s and ] water to maintain a desired pH and carbonate hardness (KH). In ] with fiber-reactive dyes, sodium carbonate (often under a name such as soda ash fixative or soda ash activator) is used as ] to ensure proper chemical bonding of the dye with cellulose (plant) fiber. It is also used in the ] to maintain a favourable ] as a float conditioner besides ] and other mildly basic compounds. | |||
| * Sodium carbonate is also used in the production of sherbet powder. The cooling and fizzing sensation results from the endothermic reaction between sodium carbonate and a weak acid, commonly citric acid, releasing carbon dioxide gas, which occurs when the sherbet is moistened by saliva. | |||
| ===Precursor to other compounds=== | |||
| *As a food additive (E500), it is used in the production of ''snus'' (Swedish-style ]) to stabilize the pH of the final product. In Sweden, ''snus'' is regulated as a food product because it is put into the mouth, requires pasteurization, and contains only ingredients that are approved as food additives. | |||
| Sodium {{em|bicarbonate}} (NaHCO<sub>3</sub>) or baking soda, also a component in fire extinguishers, is often generated from sodium carbonate. Although NaHCO<sub>3</sub> is itself an intermediate product of the Solvay process, the heating needed to remove the ammonia that contaminates it decomposes some NaHCO<sub>3</sub>, making it more economical to react finished Na<sub>2</sub>CO<sub>3</sub> with CO<sub>2</sub>: | |||
| {{block indent|Na<sub>2</sub>CO<sub>3</sub> + CO<sub>2</sub> + H<sub>2</sub>O → 2NaHCO<sub>3</sub>}} | |||
| In a related reaction, sodium carbonate is used to make ] (NaHSO<sub>3</sub>), which is used for the "sulfite" method of separating ] from cellulose. This reaction is exploited for removing ] from flue gases in power stations: | |||
| *It is used widely{{Citation needed|date=September 2011}} in China, commonly sold as a edible alkali or food-grade alkali powder (salt) in most Chinese supermarkets. Added to water, it is used to replace lye-water in the crust of traditional Cantonese ]s, and in many other Chinese steamed buns and noodles. | |||
| {{block indent|Na<sub>2</sub>CO<sub>3</sub> + SO<sub>2</sub> + H<sub>2</sub>O → NaHCO<sub>3</sub> + NaHSO<sub>3</sub>}} | |||
| This application has become more common, especially where stations have to meet stringent emission controls. | |||
| Sodium carbonate is used by the cotton industry to neutralize the sulfuric acid needed for acid delinting of fuzzy cottonseed. | |||
| It is also used to form carbonates of other metals by ion exchange, often with the other metals' sulphates. | |||
| *In casting, it is referred to as "bonding agent" and is used to allow wet alginate to adhere to gelled alginate. | |||
| ===Miscellaneous=== | |||
| *Sodium carbonate is used in toothpastes, where it acts as a foaming agent and an abrasive, and to temporarily increase mouth pH. | |||
| Sodium carbonate is used by the brick industry as a wetting agent to reduce the amount of water needed to extrude the clay. In casting, it is referred to as "bonding agent" and is used to allow wet ] to adhere to gelled alginate. Sodium carbonate is used in toothpastes, where it acts as a foaming agent and an abrasive, and to temporarily increase mouth pH. | |||
| Sodium carbonate is also used in the processing and tanning of animal hides.<ref>{{cite web|title=Home Tanning Hides and Furs|url= https://shareok.org/bitstream/handle/11244/331379/oksa_ANSI-3998_2007-06.pdf?sequence=1&isAllowed=y|access-date=16 April 2024}}</ref> | |||
| *Sodium carbonate is used to create the photo process known as reticulation. | |||
| ==Physical properties== | |||
| *Sodium carbonate may be used for safely cleaning silver. First, aluminum foil is added to a glass or ceramic container, and covered with very hot water and some sodium carbonate. Silver items are dipped into this "bath" to clean them, making sure the silver makes contact with the aluminum foil. Finally, the silver is rinsed in water and left to dry. | |||
| The integral ] of sodium carbonate is −28.1 kJ/mol for a 10% w/w aqueous solution.<ref>{{cite web|url=http://www.tatachemicals.com/north-america/product/images/fig_2_1.jpg|title=Tatachemicals.com/north-america/product/images/fig_2_1.jpg}}</ref> The ] of sodium carbonate monohydrate is 1.3.<ref name=pphoic /> | |||
| ==Occurrence== | ==Occurrence as natural mineral== | ||
| ] | |||
| Sodium carbonate crystallizes from water to form three different hydrates: | |||
| Sodium carbonate is soluble in water, and can occur naturally in arid regions, especially in mineral deposits (''evaporites'') formed when seasonal lakes evaporate. Deposits of the mineral ] have been mined from dry lake bottoms in Egypt since ancient times, when natron was used in the preparation of ] and in the early manufacture of glass. | |||
| # sodium carbonate decahydrate (]) | |||
| # sodium carbonate heptahydrate (not known in mineral form) | |||
| # sodium carbonate monohydrate (mineral thermonatrite) | |||
| Sodium carbonate is soluble in water, but can occur naturally in arid regions, especially in mineral deposits (''evaporites'') formed when seasonal lakes evaporate. Deposits of the mineral ] have been mined from dry lake bottoms in Egypt since ancient times, when natron was used in the preparation of ] and in the early manufacture of glass. | |||
| The anhydrous mineral form of sodium carbonate is quite rare and called natrite. Sodium carbonate also erupts from ], Tanzania's unique volcano, and it is presumed erupted from other volcanoes in the past but |
The anhydrous mineral form of sodium carbonate is quite rare and called natrite. Sodium carbonate also erupts from ], Tanzania's unique volcano, and it is presumed to have erupted from other volcanoes in the past, but due to these minerals' instability at the Earth's surface, are likely to be eroded. All three mineralogical forms of sodium carbonate, as well as ], trisodium hydrogendi carbonate dihydrate, are also known from ultra-alkaline ], that occur for example in the ] in Russia. | ||
| Extra terrestrially, known sodium carbonate is rare. Deposits have been identified as the source of ], interior material that has been brought to the surface.<ref>{{cite journal |title=Bright carbonate deposits as evidence of aqueous alteration on (1) Ceres |journal=Nature |date= 29 June 2016 |last=De Sanctis |first=M. C. |display-authors=etal |volume=536 |issue= 7614|doi=10.1038/nature18290 |pages=54–57 |pmid=27362221|bibcode=2016Natur.536...54D |s2cid=4465999 }}</ref> While there are ], and these are expected to include sodium carbonate,<ref name="Kargel2004">{{cite book|author=Jeffrey S. Kargel|title=Mars - A Warmer, Wetter Planet|url=https://books.google.com/books?id=0QY0U6qJKFUC&pg=PA399|date=23 July 2004|publisher=Springer Science & Business Media|isbn=978-1-85233-568-7|pages=399–}}</ref> deposits have yet to be confirmed, this absence is explained by some as being due to a global dominance of low ] in previously aqueous ].<ref>Grotzinger, J. and R. Milliken (eds.) 2012. Sedimentary Geology of Mars. SEPM</ref> | |||
| ==Production== | ==Production== | ||
| The initial large-scale chemical procedure was established in England in 1823 to manufacture soda ash.<ref name="Himmel" /> | |||
| ===Mining=== | ===Mining=== | ||
| ], also known as ] (Na<sub>3</sub>HCO<sub>3</sub>CO<sub>3</sub>·2H<sub>2</sub>O), is mined in several areas of the US and provides nearly all the US consumption of sodium carbonate. Large natural deposits found in 1938, such as the one near ], have made mining more economical than industrial production in North America. There are important reserves of trona in Turkey;<ref>{{cite news |date=2021-08-09 |title=Ciner Weighs Sale of Stake in $5 Billion Soda Ash Unit |language=en |work=Bloomberg.com |url=https://www.bloomberg.com/news/articles/2021-08-09/ciner-said-to-weigh-sale-of-stake-in-5-billion-soda-ash-unit |access-date=2023-12-04}}</ref> two million tons of soda ash have been extracted from the reserves near Ankara. | |||
| ], ] (Na<sub>3</sub>HCO<sub>3</sub>CO<sub>3</sub>·2H<sub>2</sub>O), is mined in several areas of the United States and provides nearly all the domestic sodium carbonate. Large natural deposits found in 1938, such as the one near ], have made mining more economical than industrial production in North America. | |||
| It is also mined from some alkaline lakes such as ] in Kenya by dredging. Hot saline springs continuously replenish salt in the lake so that, provided the rate of dredging is no greater than the replenishment rate, the source is fully sustainable. | |||
| ===Barilla and kelp=== | ===Barilla and kelp=== | ||
| Several "]" (salt-tolerant) plant species and seaweed species can be processed to yield an impure form of sodium carbonate, and these sources predominated in Europe and elsewhere until the early 19th century. The land plants (typically ]s or ]s) or the seaweed (typically '']'' species) were harvested, dried, and burned. The ashes were then " |
Several "]" (salt-tolerant) plant species and seaweed species can be processed to yield an impure form of sodium carbonate, and these sources predominated in Europe and elsewhere until the early 19th century. The land plants (typically ]s or ]s) or the seaweed (typically '']'' species) were harvested, dried, and burned. The ashes were then "]" (washed with water) to form an alkali solution. This solution was boiled dry to create the final product, which was termed "soda ash"; this very old name derives from the Arabic word ''soda'', in turn applied to '']'', one of the many species of seashore plants harvested for production. "Barilla" is a commercial term applied to an impure form of ] obtained from coastal plants or ].<ref>{{cite book |last1=Hooper |first1=Robert |author-link1=Robert Hooper (physician) |title=Lexicon Medicum |date=1802 |publisher=Longman |location=London |pages=1198–9 |edition=1848|oclc= 27671024}}</ref> | ||
| The sodium carbonate concentration in soda ash varied very widely, from 2–3 percent for the seaweed-derived form ("]"), to 30 percent for the best ] produced from ] plants in Spain. Plant and seaweed sources for soda ash, and also for the related ] "]", became increasingly inadequate by the end of the 18th century, and the search for commercially |
The sodium carbonate concentration in soda ash varied very widely, from 2–3 percent for the seaweed-derived form ("]"), to 30 percent for the best ] produced from ] plants in Spain. Plant and seaweed sources for soda ash, and also for the related ] "]", became increasingly inadequate by the end of the 18th century, and the search for commercially viable routes to synthesizing soda ash from salt and other chemicals intensified.<ref name="Clow52"> | ||
| Clow, Archibald and Clow, Nan L. (1952). ''Chemical Revolution |
Clow, Archibald and Clow, Nan L. (June 1952). ''Chemical Revolution''. Ayer. pp. 65–90. {{ISBN|0-8369-1909-2}}.</ref> | ||
| ===Leblanc process=== | ===Leblanc process=== | ||
| {{Main|Leblanc process}} | {{Main|Leblanc process}} | ||
| In |
In 1792, the French chemist ] patented a process for producing sodium carbonate from salt, ], ], and coal. In the first step, sodium chloride is treated with sulfuric acid in the ]. This reaction produces ] (''salt cake'') and ]: | ||
| {{block indent|2NaCl + H<sub>2</sub>SO<sub>4</sub> → Na<sub>2</sub>SO<sub>4</sub> + 2HCl}} | |||
| The salt cake and crushed ] (]) was reduced by heating with ].<ref name=Ullmann>{{cite encyclopedia|author=Christian Thieme|encyclopedia=Ullmann's Encyclopedia of Industrial Chemistry|publisher=Wiley-VCH|location=Weinheim|year=2000|doi=10.1002/14356007.a24_299|isbn = 978-3527306732|chapter = Sodium Carbonates}}</ref> This conversion entails two parts. First is the ] whereby the coal, a source of ], ] the ] to ]: | |||
| :2 ] + ] → ] + 2 ] | |||
| {{block indent|Na<sub>2</sub>SO<sub>4</sub> + 2C → Na<sub>2</sub>S + 2CO<sub>2</sub>}} | |||
| The second stage is the reaction to produce sodium carbonate and ]: | |||
| {{block indent|Na<sub>2</sub>S + CaCO<sub>3</sub> → Na<sub>2</sub>CO<sub>3</sub> + CaS}} | |||
| This mixture is called ''black ash''. The soda ash is extracted from the black ash with water. Evaporation of this extract yields solid sodium carbonate. This extraction process was termed ]. | |||
| The hydrochloric acid produced by the ] was a major source of air pollution, and the ] byproduct also presented waste disposal issues. However, it remained the major production method for sodium carbonate until the late 1880s.<ref name="Clow52"/><ref name="Kiefer">{{cite journal |last1=Kiefer |first1=David M. |date=January 2002 |url=http://pubs.acs.org/subscribe/journals/tcaw/11/i01/html/01chemchron.html |title=It was all about alkali |journal=Today's Chemist at Work |volume=11 |issue=1 |pages=45–6}}</ref> | |||
| :] + ] + 2 ] → Na<sub>2</sub>CO<sub>3</sub> + 2 ] + ] | |||
| The sodium carbonate was ] from the ashes with water, and then collected by allowing the water to evaporate. | |||
| The hydrochloric acid produced by the ] was a major source of air pollution, and the ] byproduct also presented waste disposal issues. However, it remained the major production method for sodium carbonate until the late 1880s.<ref name="Clow52"/><ref name="Kiefer"> | |||
| Kiefer, David M. (200 | |||
| s2). ''Today's Chemist at Work,'' Vol. 11, No. 1, pp. 45–6.</ref> | |||
| ===Solvay process=== | ===Solvay process=== | ||
| {{Main|Solvay process}} | {{Main|Solvay process}} | ||
| In 1861, the ] industrial chemist ] developed a method to convert sodium chloride to sodium carbonate using ]. The ] centered around a large hollow tower. At the bottom, calcium carbonate (limestone) was heated to release carbon dioxide: | |||
| In 1861, the ] industrial chemist ] developed a method for making sodium carbonate by first reacting ], ], water, and carbon dioxide to generate ] and ]:<ref name=Ullmann/> | |||
| :] → ] + ] | |||
| {{block indent|NaCl + NH<sub>3</sub> + CO<sub>2</sub> + H<sub>2</sub>O → NaHCO<sub>3</sub> + NH<sub>4</sub>Cl}} | |||
| At the top, a concentrated solution of sodium chloride and ammonia entered the tower. As the carbon dioxide bubbled up through it, sodium bicarbonate precipitated: | |||
| The resulting sodium bicarbonate was then converted to sodium carbonate by heating it, releasing water and carbon dioxide: | |||
| :] + ] + ] + ] → ] + ] | |||
| {{block indent|2NaHCO<sub>3</sub> → Na<sub>2</sub>CO<sub>3</sub> + H<sub>2</sub>O + CO<sub>2</sub>}} | |||
| The sodium bicarbonate was then converted to sodium carbonate by heating it, releasing water and carbon dioxide: | |||
| Meanwhile, the ammonia was regenerated from the ammonium chloride byproduct by treating it with the lime (]) left over from carbon dioxide generation: | |||
| :2 ] → Na<sub>2</sub>CO<sub>3</sub> + ] + ] | |||
| {{block indent|2NH<sub>4</sub>Cl + CaO → 2NH<sub>3</sub> + CaCl<sub>2</sub> + H<sub>2</sub>O}} | |||
| Meanwhile, the ammonia was regenerated from the ammonium chloride byproduct by treating it with the lime (]) left over from carbon dioxide generation: | |||
| The Solvay process recycles its ammonia. It consumes only brine and limestone, and ] is its only waste product. The process is substantially more economical than the Leblanc process, which generates two waste products, ] and ]. The Solvay process quickly came to dominate sodium carbonate production worldwide. By 1900, 90% of sodium carbonate was produced by the Solvay process, and the last Leblanc process plant closed in the early 1920s.<ref name=Ullmann/> | |||
| :] + ] → ] | |||
| :] + 2 ] → ] + 2 ] + 2 ] | |||
| The second step of the Solvay process, heating sodium bicarbonate, is used on a small scale by home cooks and in restaurants to make sodium carbonate for culinary purposes (including ] and ]). The method is appealing to such users because sodium bicarbonate is widely sold as baking soda, and the temperatures required ({{convert|250|F|C}} to {{convert|300|F|C}}) to convert baking soda to sodium carbonate are readily achieved in conventional kitchen ]s.<ref name="McGee"/> | |||
| Because the Solvay process recycles its ammonia, it consumes only brine and limestone, and has ] as its only waste product. This made it substantially more economical than the Leblanc process, and it soon came to dominate world sodium carbonate production. By 1900, 90% of sodium carbonate was produced by the Solvay process, and the last Leblanc process plant closed in the early 1920s. | |||
| ===Hou's process=== | ===Hou's process=== | ||
| This process was developed by Chinese chemist ] in the 1930s. The earlier ] by-product carbon dioxide was pumped through a saturated solution of ] and ammonia to produce sodium bicarbonate by these reactions: | |||
| {{block indent|] + 2] → ] + 4]}} | |||
| Developed by Chinese chemist ] in 1930s, the first few steps are the same as the Solvay process. However, instead of treating the remaining solution with lime, carbon dioxide and ammonia are pumped into the solution, then sodium chloride is added until the solution saturates at 40 °C. Next, the solution is cooled to 10 °C. ] precipitates and is removed by filtration, and the solution is recycled to produce more sodium carbonate. Hou's process eliminates the production of ] and the byproduct ] can be refined or used as a fertilizer. | |||
| {{block indent|3] + ] → 2]}} | |||
| {{block indent|] + ] + ] → ]}} | |||
| {{block indent|] + ] → ] + ]}} | |||
| The sodium bicarbonate was collected as a precipitate due to its low solubility and then heated up to approximately {{Convert|80|C|}} or {{Convert|95|C|}} to yield pure sodium carbonate similar to last step of the Solvay process. More sodium chloride is added to the remaining solution of ammonium and sodium chlorides; also, more ammonia is pumped at 30-40 °C to this solution. The solution temperature is then lowered to below 10 °C. Solubility of ammonium chloride is higher than that of sodium chloride at 30 °C and lower at 10 °C. Due to this temperature-dependent solubility difference and the ], ammonium chloride is precipitated in a sodium chloride solution. | |||
| The Chinese name of Hou's process, ''lianhe zhijian fa'' ({{zh|c=联合制碱法|labels=no}}), means "coupled manufacturing alkali method": Hou's process is coupled to the ] and offers better ] by eliminating the production of calcium chloride, since any ammonia generated gets used by the reaction. The by-product ammonium chloride can be sold as a fertilizer. | |||
| ==See also== | |||
| * ] | |||
| ==References== | ==References== | ||
| {{ |
{{reflist}} | ||
| ==Further reading== | |||
| * {{cite book | last1 = Eggeman | first1 = T. | chapter = Sodium Carbonate | doi = 10.1002/0471238961.1915040918012108.a01.pub3 | title = Kirk-Othmer Encyclopedia of Chemical Technology | year = 2011 | pages = 1–11 | isbn = 978-0471238966 }} | |||
| * {{cite book | last1 = Thieme | first1 = C. | chapter = Sodium Carbonates | doi = 10.1002/14356007.a24_299 | title = Ullmann's Encyclopedia of Industrial Chemistry | year = 2000 | isbn = 978-3527306732 }} | |||
| ==External links== | ==External links== | ||
| {{Commons category|Sodium carbonate}} | {{Commons category|Sodium carbonate}} | ||
| * | * | ||
| * | * | ||
| * | * | ||
| * | * | ||
| * by synthetic processes | |||
| <br />{{carbonates}} | |||
| {{Sodium compounds}} | {{Sodium compounds}} | ||
| {{Authority control}} | |||
| {{DEFAULTSORT:Sodium Carbonate}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 22:02, 5 January 2025
Chemical compound Not to be confused with Sodium bicarbonate (baking soda), a similar compound.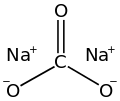
| |

| |
| |
| Names | |
|---|---|
| IUPAC name Sodium carbonate | |
| Other names Soda ash, washing soda, soda crystals, sodium trioxocarbonate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.127 |
| EC Number |
|
| E number | E500(i) (acidity regulators, ...) |
| PubChem CID | |
| RTECS number |
|
| UNII |
|
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Na2CO3 |
| Molar mass | 105.9888 g/mol (anhydrous) 286.1416 g/mol (decahydrate) |
| Appearance | White solid, hygroscopic |
| Odor | Odorless |
| Density |
|
| Melting point | 851 °C (1,564 °F; 1,124 K) (Anhydrous) 100 °C (212 °F; 373 K) decomposes (monohydrate) 33.5 °C (92.3 °F; 306.6 K) decomposes (heptahydrate) 34 °C (93 °F; 307 K) (decahydrate) |
| Solubility in water | Anhydrous, g/100 mL:
|
| Solubility | Soluble in aq. alkalis, glycerol Slightly soluble in aq. alcohol Insoluble in CS2, acetone, alkyl acetates, alcohol, benzonitrile, liquid ammonia |
| Solubility in glycerine | 98.3 g/100 g (155 °C) |
| Solubility in ethanediol | 3.46 g/100 g (20 °C) |
| Solubility in dimethylformamide | 0.5 g/kg |
| Acidity (pKa) | 10.33 |
| Magnetic susceptibility (χ) | −4.1·10 cm/mol |
| Refractive index (nD) | 1.485 (anhydrous) 1.420 (monohydrate) 1.405 (decahydrate) |
| Viscosity | 3.4 cP (887 °C) |
| Structure | |
| Crystal structure | Monoclinic (γ-form, β-form, δ-form, anhydrous) Orthorhombic (monohydrate, heptahydrate) |
| Space group | C2/m, No. 12 (γ-form, anhydrous, 170 K) C2/m, No. 12 (β-form, anhydrous, 628 K) P21/n, No. 14 (δ-form, anhydrous, 110 K) Pca21, No. 29 (monohydrate) Pbca, No. 61 (heptahydrate) |
| Point group | 2/m (γ-form, β-form, δ-form, anhydrous) mm2 (monohydrate) 2/m 2/m 2/m (heptahydrate) |
| Lattice constant | a = 8.920(7) Å, b = 5.245(5) Å, c = 6.050(5) Å (γ-form, anhydrous, 295 K)α = 90°, β = 101.35(8)°, γ = 90° |
| Coordination geometry | Octahedral (Na, anhydrous) |
| Thermochemistry | |
| Heat capacity (C) | 112.3 J/mol·K |
| Std molar entropy (S298) |
135 J/mol·K |
| Std enthalpy of formation (ΔfH298) |
−1130.7 kJ/mol |
| Gibbs free energy (ΔfG) | −1044.4 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H313+H333, H319 |
| Precautionary statements | P305+P351+P338 |
| NFPA 704 (fire diamond) |
 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 4090 mg/kg (rat, oral) |
| Safety data sheet (SDS) | MSDS |
| Related compounds | |
| Other anions | Sodium bicarbonate |
| Other cations | Lithium carbonate Potassium carbonate Rubidium carbonate Cesium carbonate |
| Related compounds | Sodium sesquicarbonate Sodium percarbonate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Sodium carbonate (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the chloralkali process.
Hydrates
Sodium carbonate is obtained as three hydrates and as the anhydrous salt:
- sodium carbonate decahydrate (natron), Na2CO3·10H2O, which readily effloresces to form the monohydrate.
- sodium carbonate heptahydrate (not known in mineral form), Na2CO3·7H2O.
- sodium carbonate monohydrate (thermonatrite), Na2CO3·H2O. Also known as crystal carbonate.
- anhydrous sodium carbonate (natrite), also known as calcined soda, is formed by heating the hydrates. It is also formed when sodium hydrogencarbonate is heated (calcined) e.g. in the final step of the Solvay process.
The decahydrate is formed from water solutions crystallizing in the temperature range −2.1 to +32.0 °C, the heptahydrate in the narrow range 32.0 to 35.4 °C and above this temperature the monohydrate forms. In dry air the decahydrate and heptahydrate lose water to give the monohydrate. Other hydrates have been reported, e.g. with 2.5 units of water per sodium carbonate unit ("Penta hemihydrate").
Washing soda
Sodium carbonate decahydrate (Na2CO3·10H2O), also known as washing soda, is the most common hydrate of sodium carbonate containing 10 molecules of water of crystallization. Soda ash is dissolved in water and crystallized to get washing soda.
It is one of the few metal carbonates that is soluble in water.
Applications
Some common applications of sodium carbonate include:
- As a cleansing agent for domestic purposes like washing clothes. Sodium carbonate is a component of many dry soap powders. It has detergent properties through the process of saponification, which converts fats and grease to water-soluble salts (specifically, soaps).
- It is used for lowering the hardness of water (see § Water softening).
- It is used in the manufacture of glass, soap, and paper (see § Glass manufacture).
- It is used in the manufacture of sodium compounds like borax (sodium borate).
Glass manufacture
Sodium carbonate serves as a flux for silica (SiO2, melting point 1,713 °C), lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass insoluble. Bottle and window glass ("soda–lime glass" with transition temperature ~570 °C) is made by melting such mixtures of sodium carbonate, calcium carbonate, and silica sand (silicon dioxide (SiO2)). When these materials are heated, the carbonates release carbon dioxide. In this way, sodium carbonate is a source of sodium oxide. Soda–lime glass has been the most common form of glass for centuries. It is also a key input for tableware glass manufacturing.
Water softening
See also: Hard water and Water softeningHard water usually contains calcium or magnesium ions. Sodium carbonate is used for removing these ions and replacing them with sodium ions.
Sodium carbonate is a water-soluble source of carbonate. The calcium and magnesium ions form insoluble solid precipitates upon treatment with carbonate ions:
Ca + CO2−3 → CaCO3 (s)The water is softened because it no longer contains dissolved calcium ions and magnesium ions.
Food additive and cooking
Sodium carbonate has several uses in cuisine, largely because it is a stronger base than baking soda (sodium bicarbonate) but weaker than lye (which may refer to sodium hydroxide or, less commonly, potassium hydroxide). Alkalinity affects gluten production in kneaded doughs, and also improves browning by reducing the temperature at which the Maillard reaction occurs. To take advantage of the former effect, sodium carbonate is therefore one of the components of kansui (かん水), a solution of alkaline salts used to give Japanese ramen noodles their characteristic flavour and chewy texture; a similar solution is used in Chinese cuisine to make lamian, for similar reasons. Cantonese bakers similarly use sodium carbonate as a substitute for lye-water to give moon cakes their characteristic texture and improve browning. In German cuisine (and Central European cuisine more broadly), breads such as pretzels and lye rolls traditionally treated with lye to improve browning can be treated instead with sodium carbonate; sodium carbonate does not produce quite as strong a browning as lye, but is much safer and easier to work with.
Sodium carbonate is used in the production of sherbet powder. The cooling and fizzing sensation results from the endothermic reaction between sodium carbonate and a weak acid, commonly citric acid, releasing carbon dioxide gas, which occurs when the sherbet is moistened by saliva.
Sodium carbonate also finds use in the food industry as a food additive (European Food Safety Authority number E500) as an acidity regulator, anticaking agent, raising agent, and stabilizer. It is also used in the production of snus to stabilize the pH of the final product.
While it is less likely to cause chemical burns than lye, care must still be taken when working with sodium carbonate in the kitchen, as it is corrosive to aluminum cookware, utensils, and foil.
Other applications
Sodium carbonate is also used as a relatively strong base in various fields. As a common alkali, it is preferred in many chemical processes because it is cheaper than sodium hydroxide and far safer to handle. Its mildness especially recommends its use in domestic applications.
For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It is also a common additive in swimming pools and aquarium water to maintain a desired pH and carbonate hardness (KH). In dyeing with fiber-reactive dyes, sodium carbonate (often under a name such as soda ash fixative or soda ash activator) is used as mordant to ensure proper chemical bonding of the dye with cellulose (plant) fiber. It is also used in the froth flotation process to maintain a favourable pH as a float conditioner besides CaO and other mildly basic compounds.
Precursor to other compounds
Sodium bicarbonate (NaHCO3) or baking soda, also a component in fire extinguishers, is often generated from sodium carbonate. Although NaHCO3 is itself an intermediate product of the Solvay process, the heating needed to remove the ammonia that contaminates it decomposes some NaHCO3, making it more economical to react finished Na2CO3 with CO2:
Na2CO3 + CO2 + H2O → 2NaHCO3In a related reaction, sodium carbonate is used to make sodium bisulfite (NaHSO3), which is used for the "sulfite" method of separating lignin from cellulose. This reaction is exploited for removing sulfur dioxide from flue gases in power stations:
Na2CO3 + SO2 + H2O → NaHCO3 + NaHSO3This application has become more common, especially where stations have to meet stringent emission controls.
Sodium carbonate is used by the cotton industry to neutralize the sulfuric acid needed for acid delinting of fuzzy cottonseed.
It is also used to form carbonates of other metals by ion exchange, often with the other metals' sulphates.
Miscellaneous
Sodium carbonate is used by the brick industry as a wetting agent to reduce the amount of water needed to extrude the clay. In casting, it is referred to as "bonding agent" and is used to allow wet alginate to adhere to gelled alginate. Sodium carbonate is used in toothpastes, where it acts as a foaming agent and an abrasive, and to temporarily increase mouth pH.
Sodium carbonate is also used in the processing and tanning of animal hides.
Physical properties
The integral enthalpy of solution of sodium carbonate is −28.1 kJ/mol for a 10% w/w aqueous solution. The Mohs hardness of sodium carbonate monohydrate is 1.3.
Occurrence as natural mineral

Sodium carbonate is soluble in water, and can occur naturally in arid regions, especially in mineral deposits (evaporites) formed when seasonal lakes evaporate. Deposits of the mineral natron have been mined from dry lake bottoms in Egypt since ancient times, when natron was used in the preparation of mummies and in the early manufacture of glass.
The anhydrous mineral form of sodium carbonate is quite rare and called natrite. Sodium carbonate also erupts from Ol Doinyo Lengai, Tanzania's unique volcano, and it is presumed to have erupted from other volcanoes in the past, but due to these minerals' instability at the Earth's surface, are likely to be eroded. All three mineralogical forms of sodium carbonate, as well as trona, trisodium hydrogendi carbonate dihydrate, are also known from ultra-alkaline pegmatitic rocks, that occur for example in the Kola Peninsula in Russia.
Extra terrestrially, known sodium carbonate is rare. Deposits have been identified as the source of bright spots on Ceres, interior material that has been brought to the surface. While there are carbonates on Mars, and these are expected to include sodium carbonate, deposits have yet to be confirmed, this absence is explained by some as being due to a global dominance of low pH in previously aqueous Martian soil.
Production
The initial large-scale chemical procedure was established in England in 1823 to manufacture soda ash.
Mining
Trona, also known as trisodium hydrogendicarbonate dihydrate (Na3HCO3CO3·2H2O), is mined in several areas of the US and provides nearly all the US consumption of sodium carbonate. Large natural deposits found in 1938, such as the one near Green River, Wyoming, have made mining more economical than industrial production in North America. There are important reserves of trona in Turkey; two million tons of soda ash have been extracted from the reserves near Ankara.
Barilla and kelp
Several "halophyte" (salt-tolerant) plant species and seaweed species can be processed to yield an impure form of sodium carbonate, and these sources predominated in Europe and elsewhere until the early 19th century. The land plants (typically glassworts or saltworts) or the seaweed (typically Fucus species) were harvested, dried, and burned. The ashes were then "lixivated" (washed with water) to form an alkali solution. This solution was boiled dry to create the final product, which was termed "soda ash"; this very old name derives from the Arabic word soda, in turn applied to Salsola soda, one of the many species of seashore plants harvested for production. "Barilla" is a commercial term applied to an impure form of potash obtained from coastal plants or kelp.
The sodium carbonate concentration in soda ash varied very widely, from 2–3 percent for the seaweed-derived form ("kelp"), to 30 percent for the best barilla produced from saltwort plants in Spain. Plant and seaweed sources for soda ash, and also for the related alkali "potash", became increasingly inadequate by the end of the 18th century, and the search for commercially viable routes to synthesizing soda ash from salt and other chemicals intensified.
Leblanc process
Main article: Leblanc processIn 1792, the French chemist Nicolas Leblanc patented a process for producing sodium carbonate from salt, sulfuric acid, limestone, and coal. In the first step, sodium chloride is treated with sulfuric acid in the Mannheim process. This reaction produces sodium sulfate (salt cake) and hydrogen chloride:
2NaCl + H2SO4 → Na2SO4 + 2HClThe salt cake and crushed limestone (calcium carbonate) was reduced by heating with coal. This conversion entails two parts. First is the carbothermic reaction whereby the coal, a source of carbon, reduces the sulfate to sulfide:
Na2SO4 + 2C → Na2S + 2CO2The second stage is the reaction to produce sodium carbonate and calcium sulfide:
Na2S + CaCO3 → Na2CO3 + CaSThis mixture is called black ash. The soda ash is extracted from the black ash with water. Evaporation of this extract yields solid sodium carbonate. This extraction process was termed lixiviating.
The hydrochloric acid produced by the Leblanc process was a major source of air pollution, and the calcium sulfide byproduct also presented waste disposal issues. However, it remained the major production method for sodium carbonate until the late 1880s.
Solvay process
Main article: Solvay processIn 1861, the Belgian industrial chemist Ernest Solvay developed a method for making sodium carbonate by first reacting sodium chloride, ammonia, water, and carbon dioxide to generate sodium bicarbonate and ammonium chloride:
NaCl + NH3 + CO2 + H2O → NaHCO3 + NH4ClThe resulting sodium bicarbonate was then converted to sodium carbonate by heating it, releasing water and carbon dioxide:
2NaHCO3 → Na2CO3 + H2O + CO2Meanwhile, the ammonia was regenerated from the ammonium chloride byproduct by treating it with the lime (calcium oxide) left over from carbon dioxide generation:
2NH4Cl + CaO → 2NH3 + CaCl2 + H2OThe Solvay process recycles its ammonia. It consumes only brine and limestone, and calcium chloride is its only waste product. The process is substantially more economical than the Leblanc process, which generates two waste products, calcium sulfide and hydrogen chloride. The Solvay process quickly came to dominate sodium carbonate production worldwide. By 1900, 90% of sodium carbonate was produced by the Solvay process, and the last Leblanc process plant closed in the early 1920s.
The second step of the Solvay process, heating sodium bicarbonate, is used on a small scale by home cooks and in restaurants to make sodium carbonate for culinary purposes (including pretzels and alkali noodles). The method is appealing to such users because sodium bicarbonate is widely sold as baking soda, and the temperatures required (250 °F (121 °C) to 300 °F (149 °C)) to convert baking soda to sodium carbonate are readily achieved in conventional kitchen ovens.
Hou's process
This process was developed by Chinese chemist Hou Debang in the 1930s. The earlier steam reforming by-product carbon dioxide was pumped through a saturated solution of sodium chloride and ammonia to produce sodium bicarbonate by these reactions:
CH4 + 2H2O → CO2 + 4H2 3H2 + N2 → 2NH3 NH3 + CO2 + H2O → NH4HCO3 NH4HCO3 + NaCl → NH4Cl + NaHCO3The sodium bicarbonate was collected as a precipitate due to its low solubility and then heated up to approximately 80 °C (176 °F) or 95 °C (203 °F) to yield pure sodium carbonate similar to last step of the Solvay process. More sodium chloride is added to the remaining solution of ammonium and sodium chlorides; also, more ammonia is pumped at 30-40 °C to this solution. The solution temperature is then lowered to below 10 °C. Solubility of ammonium chloride is higher than that of sodium chloride at 30 °C and lower at 10 °C. Due to this temperature-dependent solubility difference and the common-ion effect, ammonium chloride is precipitated in a sodium chloride solution.
The Chinese name of Hou's process, lianhe zhijian fa (联合制碱法), means "coupled manufacturing alkali method": Hou's process is coupled to the Haber process and offers better atom economy by eliminating the production of calcium chloride, since any ammonia generated gets used by the reaction. The by-product ammonium chloride can be sold as a fertilizer.
See also
References
- ^ Harper, J. P. (1936). Antipov, Evgeny; Bismayer, Ulrich; Huppertz, Hubert; Petrícek, Václav; Pöttgen, Rainer; Schmahl, Wolfgang; Tiekink, E. R. T.; Zou, Xiaodong (eds.). "Crystal Structure of Sodium Carbonate Monohydrate, Na2CO3. H2O". Zeitschrift für Kristallographie - Crystalline Materials. 95 (1): 266–273. doi:10.1524/zkri.1936.95.1.266. ISSN 2196-7105. Retrieved 2014-07-25.
- ^ Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York: D. Van Nostrand Company. p. 633.
- ^ Comey, Arthur Messinger; Hahn, Dorothy A. (February 1921). A Dictionary of Chemical Solubilities: Inorganic (2nd ed.). New York: The MacMillan Company. pp. 208–209.
- ^ Anatolievich, Kiper Ruslan. "sodium carbonate". chemister.ru. Retrieved 2014-07-25.
- ^ Pradyot, Patnaik (2003). Handbook of Inorganic Chemicals. McGraw-Hill. p. 861. ISBN 978-0-07-049439-8.
- ^ Dusek, Michal; Chapuis, Gervais; Meyer, Mathias; Petricek, Vaclav (2003). "Sodium carbonate revisited" (PDF). Acta Crystallographica Section B. 59 (3): 337–352. Bibcode:2003AcCrB..59..337D. doi:10.1107/S0108768103009017. ISSN 0108-7681. PMID 12761404. Retrieved 2014-07-25.
- ^ Betzel, C.; Saenger, W.; Loewus, D. (1982). "Sodium Carbonate Heptahydrate". Acta Crystallographica Section B. 38 (11): 2802–2804. Bibcode:1982AcCrB..38.2802B. doi:10.1107/S0567740882009996.
- ^ Sigma-Aldrich Co., Sodium carbonate. Retrieved on 2014-05-06.
- Chambers, Michael. "ChemIDplus - 497-19-8 - CDBYLPFSWZWCQE-UHFFFAOYSA-L - Sodium carbonate [NF] - Similar structures search, synonyms, formulas, resource links, and other chemical information".
- "Material Safety Data Sheet – Sodium Carbonate, Anhydrous" (PDF). conservationsupportsystems.com. ConservationSupportSystems. Retrieved 2014-07-25.
- "Soda Ash Statistics and Information". United States Geographical Survey. Retrieved 2024-03-03.
- T.W.Richards and A.H. Fiske (1914). "On the transition temperatures of the transition temperatures of the hydrates of sodium carbonate as fix points in thermometry". Journal of the American Chemical Society. 36 (3): 485–490. doi:10.1021/ja02180a003.
- A. Pabst. "On the hydrates of sodium carbonate" (PDF).
- ^ Christian Thieme (2000). "Sodium Carbonates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a24_299. ISBN 978-3527306732.
- ^ "Water Hardness Reading" (PDF). Cornell Center for Materials Research.
- ^ Himmelblau, David M.; Riggs, James B. (2022). Basic principles and calculations in chemical engineering. International series in the physical and chemical engineering sciences (Ninth ed.). Boston: Pearson. ISBN 978-0-13-732717-1.
- ^ McGee, Harold (24 September 2010). "For Old-Fashioned Flavor, Bake the Baking Soda". The New York Times. Retrieved 25 April 2019.
- "Sodium Carbonate". corrosionpedia. Janalta Interactive. Retrieved 9 November 2020.
- "Home Tanning Hides and Furs" (PDF). Retrieved 16 April 2024.
- "Tatachemicals.com/north-america/product/images/fig_2_1.jpg".
- De Sanctis, M. C.; et al. (29 June 2016). "Bright carbonate deposits as evidence of aqueous alteration on (1) Ceres". Nature. 536 (7614): 54–57. Bibcode:2016Natur.536...54D. doi:10.1038/nature18290. PMID 27362221. S2CID 4465999.
- Jeffrey S. Kargel (23 July 2004). Mars - A Warmer, Wetter Planet. Springer Science & Business Media. pp. 399–. ISBN 978-1-85233-568-7.
- Grotzinger, J. and R. Milliken (eds.) 2012. Sedimentary Geology of Mars. SEPM
- "Ciner Weighs Sale of Stake in $5 Billion Soda Ash Unit". Bloomberg.com. 2021-08-09. Retrieved 2023-12-04.
- Hooper, Robert (1802). Lexicon Medicum (1848 ed.). London: Longman. pp. 1198–9. OCLC 27671024.
- ^ Clow, Archibald and Clow, Nan L. (June 1952). Chemical Revolution. Ayer. pp. 65–90. ISBN 0-8369-1909-2.
- Kiefer, David M. (January 2002). "It was all about alkali". Today's Chemist at Work. 11 (1): 45–6.
Further reading
- Eggeman, T. (2011). "Sodium Carbonate". Kirk-Othmer Encyclopedia of Chemical Technology. pp. 1–11. doi:10.1002/0471238961.1915040918012108.a01.pub3. ISBN 978-0471238966.
- Thieme, C. (2000). "Sodium Carbonates". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a24_299. ISBN 978-3527306732.
External links
- American Natural Soda Ash Company
- International Chemical Safety Card 1135
- FMC Wyoming Corporation
- Use of sodium carbonate in dyeing
- Sodium carbonate manufacturing by synthetic processes
| Compounds containing the carbonate group | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sodium compounds | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inorganic |
| ||||||||||||||
| Organic | |||||||||||||||
