| Revision as of 19:37, 1 October 2016 editForever elementary student (talk | contribs)Extended confirmed users4,688 edits →Medical Use← Previous edit | Latest revision as of 11:59, 16 January 2024 edit undoKku (talk | contribs)Extended confirmed users115,682 editsm link neurotoxicity | ||
| (57 intermediate revisions by 31 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Drugbox | {{Drugbox | ||
| | Verifiedfields = changed | |||
| | verifiedrevid = 447808729 | | verifiedrevid = 447808729 | ||
| | IUPAC_name = (''OC''-6-43)-bis(acetato)amminedichloro(cyclohexylamine)platinum | | IUPAC_name = (''OC''-6-43)-bis(acetato)amminedichloro(cyclohexylamine)platinum | ||
| Line 29: | Line 31: | ||
| | ATC_suffix = XA04 | | ATC_suffix = XA04 | ||
| | PubChem = 123974 | | PubChem = 123974 | ||
| | ChEBI_Ref = {{ebicite| |
| ChEBI_Ref = {{ebicite|changed|EBI}} | ||
| | ChEBI = 85609 | | ChEBI = 85609 | ||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| Line 38: | Line 40: | ||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=10 | H=22 | Cl=2 | N=2 | O=4 | Pt=1 | | C=10 | H=22 | Cl=2 | N=2 | O=4 | Pt=1 | ||
| | molecular_weight = 500.277 g/mol | |||
| | synonyms = BMY 45594<br>BMS 182751<br>(''OC''-6-43)-bis(acetato)amminedichlorocyclohexylamine platinum(IV) | | synonyms = BMY 45594<br>BMS 182751<br>(''OC''-6-43)-bis(acetato)amminedichlorocyclohexylamine platinum(IV) | ||
| | SMILES = Cl(Cl)(OC(=O)C)(OC(=O)C)()C1CCCCC1 | |||
| }} | }} | ||
| '''Satraplatin''' (], codenamed '''JM216''') is a ] agent that |
'''Satraplatin''' (], codenamed '''JM216''') is a ] agent that was under investigation as a treatment of patients with advanced ] who have failed previous ]. It has not yet received approval from the U.S. ].<ref>{{cite journal | vauthors = Wheate NJ, Walker S, Craig GE, Oun R | title = The status of platinum anticancer drugs in the clinic and in clinical trials | journal = Dalton Transactions | volume = 39 | issue = 35 | pages = 8113–8127 | date = September 2010 | pmid = 20593091 | doi = 10.1039/C0DT00292E | hdl-access = free | s2cid = 205766376 | hdl = 2123/14271 | author-link1 = Nial J. Wheate }}</ref> First mentioned in the medical literature in 1993,<ref>{{cite journal | vauthors = Kelland LR, Abel G, McKeage MJ, Jones M, Goddard PM, Valenti M, Murrer BA, Harrap KR | display-authors = 6 | title = Preclinical antitumor evaluation of bis-acetato-ammine-dichloro-cyclohexylamine platinum(IV): an orally active platinum drug | journal = Cancer Research | volume = 53 | issue = 11 | pages = 2581–2586 | date = June 1993 | pmid = 8388318 | url = http://cancerres.aacrjournals.org/cgi/reprint/53/11/2581.pdf | author-link8 = Kenneth Harrap }}</ref> satraplatin is the first orally active platinum-based chemotherapeutic drug;<ref name = ChoyParkYao>{{cite journal | vauthors = Choy H, Park C, Yao M | title = Current status and future prospects for satraplatin, an oral platinum analogue | journal = Clinical Cancer Research | volume = 14 | issue = 6 | pages = 1633–1638 | date = March 2008 | pmid = 18347164 | doi = 10.1158/1078-0432.CCR-07-2176 | doi-access = free }}</ref> other available platinum analogues—], ], and ]—must be given ]. | ||
| ⚫ | The drug has also been used in the treatment of lung and ovarian cancers. The proposed mode of action is that the compound binds to the ] of cancer cells rendering them incapable of ].<ref> {{webarchive|url=https://web.archive.org/web/20070704183648/http://www.spectrumpharm.com/satraplatin.html |date=2007-07-04 }}</ref> | ||
| It is made available in the United States jointly by ] and GPC Biotech under the name SPERA (SatraPlatin Expanded Rapid Access). | |||
| == Mode of action == | |||
| ⚫ | The drug has also been used in the treatment of lung and ovarian cancers. The proposed mode of action is that the compound binds to the ] of cancer cells rendering them incapable of ].<ref></ref> | ||
| The proposed mode of action is that the compound binds to the ] of cancer cells rendering them incapable of ]. In addition some cisplatin resistant tumour cell lines were sensitive to satraplatin treatment in vitro. This may be due to an altered mechanism of cellular uptake (satraplatin by passive diffusion instead of active transport for e.g. cisplatin). | |||
| == |
== Clinical development == | ||
| Satraplatin |
Satraplatin has been developed for the treatment of men with castrate-refractory, metastatic prostate cancer for several reasons. Its relative ease of administration, potential lack of cross-resistance with other platinum agents, clinical benefits seen in early studies of prostate cancer, and an unmet need in this patient population after ] failure at that time. The only Phase III trial with satraplatin (SPARC Trial) was conducted in pretreated metastatic castrate-resistant prostate cancer (CRPC), revealing a 33% reduction in risk of progression or death versus a placebo.<ref>{{cite journal | vauthors = Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, Ferrero JM, Eymard JC, Falcon S, Calabrò F, James N, Bodrogi I, Harper P, Wirth M, Berry W, Petrone ME, McKearn TJ, Noursalehi M, George M, Rozencweig M | display-authors = 6 | title = Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial | journal = Journal of Clinical Oncology | volume = 27 | issue = 32 | pages = 5431–5438 | date = November 2009 | pmid = 19805692 | doi = 10.1200/JCO.2008.20.1228 | doi-access = free }}</ref> However, no difference in overall survival was observed. An FDA or ]-approved indication has not yet been achieved. | ||
| Satraplatin appears to have clinical activity against a variety of malignancies such as ], ] and ]. | |||
| Satraplatin has a favorable toxicity profile, and appears to have clinical activity against a variety of malignancies such as ], ] and ]. The oral route of administration and the intermittent schedule makes it very convenient for clinical use. Despite this, a FDA-approved indication has not yet been achieved. The only Phase III trial with satraplatin was conducted in pre-treated metastatic prostate cancer(CRPC), revealing an improvement in progression-free survival but no overall survival benefit.<ref name=":1">''Vaishampayan UN. (2009) " "'' '';18(11):10.1517/13543780903362437. doi:10.1517/13543780903362437.''</ref> | |||
| Especially in combination with radiotherapy it appears to have good efficacy in combination for lung and squamous head and neck cancer. In a phase I study from Vanderbilt University, seven of eight patients with squamous cell carcinoma of the head and neck, who were treated with 10 to 30 mg of satraplatin thrice a week concurrently with radiotherapy achieved a complete response.<ref>{{Cite journal| vauthors = Cmelak AJ, Choy H, Murphy BA, DeVore R, Bria B, Day T, Porte K, Johnson D | display-authors = 6 |date=|title=Phase I study of JM-216 with concurrent radiation in non-small cell lung cancer and squamous cell head and neck cancer|url=|journal=Proc Am Soc Clin Oncol|volume=1999|pages=|via=}}</ref> | |||
| ⚫ | |||
| ], diarrhea, constipation, nausea or vomiting, increase risk of infection, bruising.<ref name=":1" /> | |||
| ⚫ | == Side effects == | ||
| ⚫ | |||
| Satraplatin is similar in toxicity profile to carboplatin, with no nephrotoxicity, ], or ototoxicity observed. Moreover, it is much better tolerated than cisplatin and does not require hydration for each dose. A somewhat more intense hematotoxicity is observed. ], diarrhea, constipation, nausea or vomiting, increase risk of infection, bruising.<ref name=":1">{{cite journal | vauthors = Bhargava A, Vaishampayan UN | title = Satraplatin: leading the new generation of oral platinum agents | journal = Expert Opinion on Investigational Drugs | volume = 18 | issue = 11 | pages = 1787–1797 | date = November 2009 | pmid = 19888874 | pmc = 3856359 | doi = 10.1517/13543780903362437 }}</ref> | |||
| ⚫ | == Possible risks and complications == | ||
| * ]: Cancer can increase the risk of developing a blood clot, and chemotherapy may increase this risk further. A blood clot may cause symptoms such as pain, redness and swelling in a leg, or breathlessness and chest pain. Most clots can be treated with drugs that thin the blood. | * ]: Cancer can increase the risk of developing a blood clot, and chemotherapy may increase this risk further. A blood clot may cause symptoms such as pain, redness and swelling in a leg, or breathlessness and chest pain. Most clots can be treated with drugs that thin the blood. | ||
| * ]: Satraplatin can |
* ]: Satraplatin can affect a person's ability to become pregnant and may cause sterility in men. | ||
| * Use ]: Satraplatin may harm a developing baby. It |
* Use ]: Satraplatin may harm a developing baby. It is important to use effective contraception while taking this drug and for at least a few months afterwards<ref name=":1" /> | ||
| == History == | |||
| Platinum-based chemotherapy was an accidental discovery in the laboratory by ]. Rosenberg noticed inhibited cell division of ] cells during electrolysis of a platinum-based electrode. The compound discovered was later named ] and clinically used by 1970. After years of use, cisplatin became an invaluable component of therapy for some common cancer tumors such as lung, head, neck, and bladder cancers. Kidney damage, neurotoxicity, ototoxicity, development of resistance were some of the more serious side effects that prompted the development of novel platinum analogues.<ref name=":0">''Vaishampayan UN. (2009) "Satraplatin: Leading the new generation of oral platinum agents." Expert opinion on investigational drugs. ;18(11):10.1517/13543780903362437. doi:10.1517/13543780903362437.''</ref> | |||
| In 1982 ] was found to be a popular platinum-based compound very similar to cisplatin. After many trials and analyses with successful results equivalent to those of cisplatin but with a more tolerable toxicity profile making the drug more desirable for therapy. The more serious side effects that were associated with carboplatin were bone marrow suppression and principally thrombocytopenia. Carboplatin was approved by the FDA in 1989 to be used to treat advanced ].<ref name=":0" /> | |||
| == Detailed mechanism of action == | |||
| Research found that ] was demonstrating successful activity in patients with advanced colon cancer. Studies done in 2000 to 2004 supported the utility of oxaliplatin in combination with chemotherapy. The use of oxaliplatin in combination with ] and ] was approved by the FDA for the treatment of only stage IV ] then later approved for stage III colon cancer patients who had previously received complete resection of the primary tumor.<ref name=":0" /> | |||
| Many human tumors including testicular, bladder, lung, head, neck, and cervical cancers have been treated with platinum compounds. All of the marketed platinum analogues must be administered via intravenous infusion is one of the main disadvantages for these platinum compounds due to severe, dose |
Many human tumors including testicular, bladder, lung, head, neck, and cervical cancers have been treated with platinum compounds.<ref name=":1" /> All of the marketed platinum analogues must be administered via intravenous infusion is one of the main disadvantages for these platinum compounds due to severe, dose-limiting effects. An acquired resistance to cisplatin/carboplatin in ovarian cancer was discovered due to insufficient amounts of platinum reaching the target DNA or failure to achieve cell death. These drawbacks led to the development of the next generation of platinum analogues such as satraplatin<ref name=":1" /> | ||
| ⚫ | Satraplatin is a prodrug, meaning it is metabolized in the body and transformed into its working form. The two polar acetate groups on satraplatin increase the drugs ], which in turn allows for a large fraction of the administered dose to make it into the bloodstream where metabolism begins. Once the molecule makes it to the bloodstream the drug loses its acetate groups. At this point the drug is structurally similar to ] with the exception of one cyclohexylamine group in place of an amine group. Since the drug is now structurally similar to cisplatin its mechanism of action is also very similar. The chlorine atoms are displaced and the platinum atom in the drug binds to guanine residues in DNA. This unfortunately happens to not only cancer cells but other regular functioning cells as well causing some of the harsh side effects. By binding to guanine residues satraplatin inhibits DNA replication and transcription which leads to subsequent ]. Where satraplatin differs is its cyclohexamine group. In cisplatin the two amine groups are symmetrical while satraplatin's cyclohexamine makes it asymmetrical which contributes to some of the drug's special properties.<ref name="ChoyParkYao"/> | ||
| == Mechanism of Action == | |||
| ⚫ | A large problem with cisplatin and other platinum based anti-cancer drugs is that the body can develop resistance to them. A major way that this happens is through a mammalian nucleotide excision repair pathway which repairs damaged DNA. However, some studies show that satraplatin compared to other platinum anti-cancer drugs can be elusive and are not recognized by the DNA repair proteins due to the different adducts on the molecule (cyclohexamine). Since satraplatin is not recognized by the DNA repair proteins the DNA remains damaged, the DNA cannot be replicated, the cell dies, and the problem of resistance is solved.<ref name = ChoyParkYao /> | ||
| ⚫ | Satraplatin is a prodrug, meaning |
||
| In vitro experiments have shown that satraplatin is more effective in well-defined hematological cancers than cisplatin. ] deficiency and ] gene mutation were identified as biomarkers of enhanced efficacy.<ref>{{cite journal | vauthors = Zander T, Xue J, Markson G, Dahm F, Renner C | title = Satraplatin Demonstrates High Cytotoxic Activity Against Genetically Defined Lymphoid Malignancies | journal = Anticancer Research | volume = 42 | issue = 4 | pages = 1821–1832 | date = April 2022 | pmid = 35347000 | doi = 10.21873/anticanres.15658 | s2cid = 247765144 | doi-access = free }}</ref> | |||
| ⚫ | A large problem with cisplatin and other platinum based anti-cancer drugs is that the body can develop resistance to them. A major way that this happens is through a mammalian nucleotide excision repair pathway which repairs damaged DNA. However, some studies show that satraplatin compared to other platinum anti-cancer drugs can be elusive and are not recognized by the DNA repair proteins due to the different adducts on the molecule (cyclohexamine). Since satraplatin is not recognized by the DNA repair proteins the DNA remains damaged, the DNA cannot be replicated, the cell dies, and the problem of resistance is solved.<ref name= |
||
| ==References== | == References == | ||
| {{Reflist}} | |||
| <references /> | |||
| {{Chemotherapeutic agents}} | {{Chemotherapeutic agents}} | ||
| {{Platinum compounds}} | {{Platinum compounds}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
Latest revision as of 11:59, 16 January 2024
Chemical compound Pharmaceutical compound | |
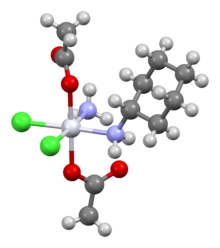 | |
| Clinical data | |
|---|---|
| Other names | BMY 45594 BMS 182751 (OC-6-43)-bis(acetato)amminedichlorocyclohexylamine platinum(IV) |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H22Cl2N2O4Pt |
| Molar mass | 500.28 g·mol |
| 3D model (JSmol) | |
SMILES
| |
| (what is this?) (verify) | |
Satraplatin (INN, codenamed JM216) is a platinum-based antineoplastic agent that was under investigation as a treatment of patients with advanced prostate cancer who have failed previous chemotherapy. It has not yet received approval from the U.S. Food and Drug Administration. First mentioned in the medical literature in 1993, satraplatin is the first orally active platinum-based chemotherapeutic drug; other available platinum analogues—cisplatin, carboplatin, and oxaliplatin—must be given intravenously.
The drug has also been used in the treatment of lung and ovarian cancers. The proposed mode of action is that the compound binds to the DNA of cancer cells rendering them incapable of dividing.
Mode of action
The proposed mode of action is that the compound binds to the DNA of cancer cells rendering them incapable of dividing. In addition some cisplatin resistant tumour cell lines were sensitive to satraplatin treatment in vitro. This may be due to an altered mechanism of cellular uptake (satraplatin by passive diffusion instead of active transport for e.g. cisplatin).
Clinical development
Satraplatin has been developed for the treatment of men with castrate-refractory, metastatic prostate cancer for several reasons. Its relative ease of administration, potential lack of cross-resistance with other platinum agents, clinical benefits seen in early studies of prostate cancer, and an unmet need in this patient population after Docetaxel failure at that time. The only Phase III trial with satraplatin (SPARC Trial) was conducted in pretreated metastatic castrate-resistant prostate cancer (CRPC), revealing a 33% reduction in risk of progression or death versus a placebo. However, no difference in overall survival was observed. An FDA or EMA-approved indication has not yet been achieved.
Satraplatin appears to have clinical activity against a variety of malignancies such as Breast Cancer, Prostate cancer and Lung cancer.
Especially in combination with radiotherapy it appears to have good efficacy in combination for lung and squamous head and neck cancer. In a phase I study from Vanderbilt University, seven of eight patients with squamous cell carcinoma of the head and neck, who were treated with 10 to 30 mg of satraplatin thrice a week concurrently with radiotherapy achieved a complete response.
Side effects
Satraplatin is similar in toxicity profile to carboplatin, with no nephrotoxicity, neurotoxicity, or ototoxicity observed. Moreover, it is much better tolerated than cisplatin and does not require hydration for each dose. A somewhat more intense hematotoxicity is observed. Anemia, diarrhea, constipation, nausea or vomiting, increase risk of infection, bruising.
Possible risks and complications
- Thrombus: Cancer can increase the risk of developing a blood clot, and chemotherapy may increase this risk further. A blood clot may cause symptoms such as pain, redness and swelling in a leg, or breathlessness and chest pain. Most clots can be treated with drugs that thin the blood.
- Fertility: Satraplatin can affect a person's ability to become pregnant and may cause sterility in men.
- Use Contraception: Satraplatin may harm a developing baby. It is important to use effective contraception while taking this drug and for at least a few months afterwards
Detailed mechanism of action
Many human tumors including testicular, bladder, lung, head, neck, and cervical cancers have been treated with platinum compounds. All of the marketed platinum analogues must be administered via intravenous infusion is one of the main disadvantages for these platinum compounds due to severe, dose-limiting effects. An acquired resistance to cisplatin/carboplatin in ovarian cancer was discovered due to insufficient amounts of platinum reaching the target DNA or failure to achieve cell death. These drawbacks led to the development of the next generation of platinum analogues such as satraplatin
Satraplatin is a prodrug, meaning it is metabolized in the body and transformed into its working form. The two polar acetate groups on satraplatin increase the drugs bioavailability, which in turn allows for a large fraction of the administered dose to make it into the bloodstream where metabolism begins. Once the molecule makes it to the bloodstream the drug loses its acetate groups. At this point the drug is structurally similar to cisplatin with the exception of one cyclohexylamine group in place of an amine group. Since the drug is now structurally similar to cisplatin its mechanism of action is also very similar. The chlorine atoms are displaced and the platinum atom in the drug binds to guanine residues in DNA. This unfortunately happens to not only cancer cells but other regular functioning cells as well causing some of the harsh side effects. By binding to guanine residues satraplatin inhibits DNA replication and transcription which leads to subsequent apoptosis. Where satraplatin differs is its cyclohexamine group. In cisplatin the two amine groups are symmetrical while satraplatin's cyclohexamine makes it asymmetrical which contributes to some of the drug's special properties.
A large problem with cisplatin and other platinum based anti-cancer drugs is that the body can develop resistance to them. A major way that this happens is through a mammalian nucleotide excision repair pathway which repairs damaged DNA. However, some studies show that satraplatin compared to other platinum anti-cancer drugs can be elusive and are not recognized by the DNA repair proteins due to the different adducts on the molecule (cyclohexamine). Since satraplatin is not recognized by the DNA repair proteins the DNA remains damaged, the DNA cannot be replicated, the cell dies, and the problem of resistance is solved.
In vitro experiments have shown that satraplatin is more effective in well-defined hematological cancers than cisplatin. MTAP deficiency and Bcl-2 gene mutation were identified as biomarkers of enhanced efficacy.
References
- Wheate NJ, Walker S, Craig GE, Oun R (September 2010). "The status of platinum anticancer drugs in the clinic and in clinical trials". Dalton Transactions. 39 (35): 8113–8127. doi:10.1039/C0DT00292E. hdl:2123/14271. PMID 20593091. S2CID 205766376.
- Kelland LR, Abel G, McKeage MJ, Jones M, Goddard PM, Valenti M, et al. (June 1993). "Preclinical antitumor evaluation of bis-acetato-ammine-dichloro-cyclohexylamine platinum(IV): an orally active platinum drug" (PDF). Cancer Research. 53 (11): 2581–2586. PMID 8388318.
- ^ Choy H, Park C, Yao M (March 2008). "Current status and future prospects for satraplatin, an oral platinum analogue". Clinical Cancer Research. 14 (6): 1633–1638. doi:10.1158/1078-0432.CCR-07-2176. PMID 18347164.
- Satraplatin — Spectrum Pharmaceuticals Archived 2007-07-04 at the Wayback Machine
- Sternberg CN, Petrylak DP, Sartor O, Witjes JA, Demkow T, Ferrero JM, et al. (November 2009). "Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial". Journal of Clinical Oncology. 27 (32): 5431–5438. doi:10.1200/JCO.2008.20.1228. PMID 19805692.
- Cmelak AJ, Choy H, Murphy BA, DeVore R, Bria B, Day T, et al. "Phase I study of JM-216 with concurrent radiation in non-small cell lung cancer and squamous cell head and neck cancer". Proc Am Soc Clin Oncol. 1999.
- ^ Bhargava A, Vaishampayan UN (November 2009). "Satraplatin: leading the new generation of oral platinum agents". Expert Opinion on Investigational Drugs. 18 (11): 1787–1797. doi:10.1517/13543780903362437. PMC 3856359. PMID 19888874.
- Zander T, Xue J, Markson G, Dahm F, Renner C (April 2022). "Satraplatin Demonstrates High Cytotoxic Activity Against Genetically Defined Lymphoid Malignancies". Anticancer Research. 42 (4): 1821–1832. doi:10.21873/anticanres.15658. PMID 35347000. S2CID 247765144.
| Platinum compounds | |||
|---|---|---|---|
| Pt(−II) | |||
| Pt(0) | |||
| Pt(II) |
| ||
| Pt(IV) | |||
| Pt(V) | |||
| Pt(VI) | |||