This is an old revision of this page, as edited by Lamro (talk | contribs) at 11:21, 15 May 2011 (links). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 11:21, 15 May 2011 by Lamro (talk | contribs) (links)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)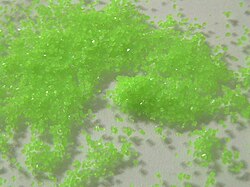
| |
| Names | |
|---|---|
| Other names Praseodymium sulphate | |
| Identifiers | |
| CAS Number | |
| ECHA InfoCard | 100.030.553 |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | Pr2(SO4)3 |
| Molar mass | 570.0031 g/mol 714.12534 g/mol (octahydrate) |
| Appearance | green crystalline solid |
| Density | g/cm |
| Melting point | 931°C |
| Boiling point | 3127°C |
| Solubility in water | 113.0 g/l (20°C) 108.8 g/l (25°C) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Xi |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Praseodymium carbonate Praseodymium chloride |
| Other cations | Neodymium sulfate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Praseodymium sulfate is a Pr compound with formula Pr2(SO4)3. It is an odourless whitish-green crystalline compound. The anhydrous substance is somewhat hygroscopic. It has three well-defined hydrates: praseodymium dihydrate, praseodymium pentahydrate, and praseodymium octahydrate. When crystallized from aqueous solutions, the octahydrate is formed as green transparent crystals.
Properties
Praseodymium sulfate is stable under standard conditions. At elevated temperatures, it gradually looses any crystal water present, whereas its colour becomes more whitish. Like all rare earth sulfates, its solubility decreases with temperature, a property once used to separate it from other, non-rare earth compounds.
| This article does not cite any sources. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Praseodymium(III) sulfate" – news · newspapers · books · scholar · JSTOR (November 2009) (Learn how and when to remove this message) |
| Praseodymium compounds | |||
|---|---|---|---|
| Pr(II) | |||
| Pr(III) |
| ||
| Pr(III,IV) | |||
| Pr(IV) | |||
| Pr(V) | |||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |