This is an old revision of this page, as edited by CitationCleanerBot (talk | contribs) at 23:15, 10 September 2011 (Various citation & identifier cleanup, plus AWB genfixes. Report errors and suggestions at User talk:CitationCleanerBot. using AWB). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 23:15, 10 September 2011 by CitationCleanerBot (talk | contribs) (Various citation & identifier cleanup, plus AWB genfixes. Report errors and suggestions at User talk:CitationCleanerBot. using AWB)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound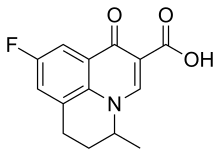 | |
| Clinical data | |
|---|---|
| Other names | 9-Fluoro-6,7-dihydro-5-methyl-1-oxo-1H,5H-benzo-quinolizine-2-carboxylic acid |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | urine and feces |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.050.857 |
| Chemical and physical data | |
| Formula | C14H12FNO3 |
| Molar mass | 261.25 g/mol g·mol |
| Melting point | 253 to 255 °C (487 to 491 °F) |
| (verify) | |
Flumequine is a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class used to treat bacterial infections. It is a first-generation fluoroquinolone antibacterial that has been removed from clinical use and is no longer being marketed. It kills bacteria by interfering with the enzymes that cause DNA to unwind and duplicate. Flumequine was used in veterinarian medicine for the treatment of enteric infections (all infections of the intestinal tract), as well as to treat cattle, swine, chickens, and fish, but only in a limited number of countries. It was occasionally used in France (and a few other European Countries) to treat urinary tract infections under the trade name Apurone. However this was a limited indication because only minimal serum levels were achieved.
History
The first quinolone used was nalidixic acid (was marketed in many countries as Negram) followed by the fluoroquinolone flumequine. The first-generation fluoroquinolone agents, such as flumequine, had poor distribution into the body tissues and limited activity. As such they were used mainly for treatment of urinary tract infections. Flumequine (benzo quinolizine) was first patented in 1973, (German Patent) by Rikker Labs. Flumequine is a known antimicrobial compound described and claimed in U.S. Pat. No. 3,896,131 (Example 3), July 22, 1975. Flumequine is the first quinolone compound with a fluorine atom at the C6-position of the related quinolone basic molecular structure. Even though this was the first fluoroquinolone, it is often times overlooked when classifying the drugs within this class by generations and excluded from such a list.
Though used frequently to treat farm animals and on occasion household pets, flumequine was also used to treat urinary tract infections in humans. Flumequine, was used transiently treat urinary infections until ocular toxicity was reported. as well as liver damage and anaphylactic shock.
In 2008, the United States Food and Drug Administration (FDA) requested that all quinolone/fluoroquinolone drugs package inserts include a Black Boxed Warning concerning the risk of spontaneous tendon ruptures, which would have included flumequine. The FDA also requested that the manufacturers send out Dear Doctor Letters regarding this new warning. Such tendon problems have also been associated with flumequine.
Drug residue
The use of flumequine in food animals had sparked considerable debate. Significant and harmful residues of quinolones have been found in animals treated with quinolones and later slaughtered and sold as food products. There has been significant concern regarding the amount of flumequine residue found within food animals such as fish, poultry and cattle. In 2003 the Joint FAO/WHO Committee on Food Additives (JECFA) withdrew the maximum residue limits (MRLs) for flumequine and carbadox based on evidence showing both are direct acting genotoxic carcinogens, therefore the Committee was unable to establish an Acceptable Daily Intake (ADI) for human exposure to such residues. The role of JECFA is to evaluate toxicology, residue chemistry and related information and make recommendations for acceptable daily intake (ADI) levels and maximum residue limits (MRLs). At its 16th session, held May 2006, the Committee on Residues of Veterinary Drugs in Foods (CCRVDF) requested information on registered uses of flumequine. As the CCRVDF did not receive any information regarding the registered uses of flumequine that they had requested, the committee members agreed to discontinue work on the MRLs for flumequine in shrimp.
Licensed uses
Urinary tract infections (veterinary and human)
Availability
Veterinary use:
- Solution; Oral; 20% (prescription only)
- Solution; Oral; 10% (prescription only)
Human use:
- Tablet; Oral; Flumequine 400 mg (discontinued)
Mode of action
Ciprofloxacin is a broad-spectrum antibiotic that is active against both Gram-positive and Gram-negative bacteria. It functions by inhibiting DNA gyrase, a type II topoisomerase, and topoisomerase IV, enzymes necessary to separate bacterial DNA, thereby inhibiting cell division.
This mechanism can also affect mammalian cell replication. In particular, some congeners of this drug family (for example those that contain the C-8 fluorine), display high activity not only against bacterial topoisomerases, but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and in vivo tumor models.
Although quinolones are highly toxic to mammalian cells in culture, its mechanism of cytotoxic action is not known. Quinolone induced DNA damage was first reported in 1986 (Hussy et al.).
Recent studies have demonstrated a correlation between mammalian cell cytotoxicity of the quinolones and the induction of micronuclei.
As such, some fluoroquinolones may cause injury to the chromosome of eukaryotic cells.
There continues to be considerable debate as to whether or not this DNA damage is to be considered one of the mechanisms of action concerning the severe adverse reactions experienced by some patients following fluoroquinolone therapy.
Adverse reactions
Flumequine was associated with severe ocular toxicity, which precluded its use in human patients. Drug-induced calculi (kidney stones) has been associated with such therapy as well. Anaphylactic shock induced by flumequine therapy has also been associated with its use. Anaphylactoid reactions such as shock, urticaria, and Quincke’s oedema have been reported to generally appear within two hours after taking the first tablet. There were eighteen reports listed within the WHO file in 1996. As with all drugs within this class, flumequine therapy may result in severe central nervous system (CNS) reactions, phototoxicity resulting in skin reactions like erythema, pruritus, urticaria and severe rashes, gastrointestinal and neurological disorders.
History of the black box warnings
See also: Quinolone § Black box warningsMusculoskeletal disorders attributed to use of quinolone antibiotics were first reported in the medical literature in 1972, as an adverse reaction to nalidixic acid. Rheumatic disease after use of a fluoroquinolone (norfloxacin) was first reported eleven years later. In response to a 1995 letter published in the New England Journal of Medicine, representatives of the U.S. Food and Drug Administration (FDA) stated that the agency would "update the labeling for all marketed fluoroquinolones to include a warning about the possibility of tendon rupture."
By August 1996, the FDA had not taken action, and the consumer advocacy group Public Citizen filed a petition with the FDA, prompting the agency to act. Two months later, the FDA published an alert in the FDA Medical Bulletin and requested that fluoroquinolone package inserts be amended to include information on this risk.
Nine years later, in 2005, the Illinois Attorney General filed a second petition with the FDA, again seeking Black Box Warnings and "Dear Doctor" letters emphasizing the risk of tendon rupture; the FDA responded that it had not yet been able to reach a decision on the matter. In 2006, Public Citizen, supported by the Illinois Attorney General, renewed its demand of ten years prior for Black Box Warnings by filing a third petition requesting such changes be made. When the FDA failed to respond to these two petitions as required by law Public Citizen, in January 2008, filed suit to compel the FDA to respond to their 2006 petition. On July 7, 2008 the FDA requested that the makers of systemic-use fluoroquinolones add a boxed warning regarding spontaneous tendon ruptures, and to develop a Medication Guide for patients. The package inserts for Ciprofloxacin, Avelox (moxifloxacin), Proquin XR, Factive (gemifloxacin), Floxin (ofloxacin), Noroxin (norfloxacin) and Levaquin (levofloxacin) were amended on September 8, 2008 to include these new warnings. Bayer, which manufactures Cipro, Avelox and Proquin XR, issued a Dear Healthcare Professional letter on October 22 concerning these changes. Ortho-McNeil, the manufacturers of Levaquin, issued a similar letter in November. through the Health Care Notification Network, a registration-only website that distributes drug alerts to licensed healthcare professionals.
Review of the FDA website indicates that the majority of the generic versions of the fluoroquinolones have not been updated to include this Black Box Warning as of September 2009. In addition, there are numerous reports that claim that this information has not been dessiminated to the pharmacist, the name brand products continue to contain the previous labels that are absent of this warning, and the Medication Guide has not been made available to the pharmicist or physician for distribution.
Drug interactions
Flumequine was found to have no effect on theophylline pharmacokinetics.
Chemistry
Flumequine is a 9-fluoro-6,7-dihydro-5-methyl-1-oxo-1H,5H-benzoquinolizine-2-carboxylic acid. The molecular formula is C14H12FNO3 It is a white powder, odorless, flavorless, insoluble in water but soluble in organic solvent.
Pharmacokinetics
Flumequine is considered to be well absorbed and is excreted in the urine and feces as the glucuronide conjugates of the parent drug and 7-hydroxyflumequine. It is eliminated within 168 hours post-dosing. However, studies concerning the calf liver showed additional unidentified residues, of which a new metabolite, ml, represented the major single metabolite 24 hours after the last dose and at all subsequent time points. The metabolite ml, which exhibited no antimicrobial activity, was present in both free and protein-bound fractions. The major residue found in the edible tissues of sheep, pigs, and chickens was parent drug together with minor amounts of the 7-hydroxy-metabolite. The only detected residue in trout was the parent drug.
See also
References
- INN: Lomefloxacin Hydrochloride
- Nelson JM, Chiller TM, Powers JH, Angulo FJ (2007). "Fluoroquinolone-resistant Campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story". Clinical Infectious Diseases. 44 (7): 977–80. doi:10.1086/512369. PMID 17342653.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Kawahara S (1998). "". Nippon Rinsho (in Japanese). 56 (12): 3096–9. PMID 9883617.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Quinolones and fluoroquinolones". Pharmacorama. Retrieved 2010-04-04.
- Francis, Philip G.; Robert J. Wells (1998). "Flumequine". in Joint FAO/WHO Expert Committee on Food Additives. Residues of some veterinary drugs in animals and foods. Rome: Food and Agriculture Organization. ISBN 92-5-104128-8. OCLC 39798999.
- Cattle only in Europe and Latin America.(Limited use in Latin America), Poultry only in Europe, Asia and Latin America, Fish only in Asia http://www.fda.gov/ohrms/dockets/dailys/03/Aug03/081403/03n-0324-bkg0001-10-tab7-vol2.pdf
- Quinalones are often used to treat severe cases of human infection with Campylobacter spp., and they are also used in veterinary medicine, especially for treating poultry. http://www.fda.gov/ohrms/dockets/dailys/02/Jan02/011102/00n-1571_c000191_06_Exhibit_05_vol25.pdf
- ^ Schena FP, Gesualdo L, Caracciolo G (1988). "A multicentre study of flumequine in the treatment of urinary tract infections". The Journal of Antimicrobial Chemotherapy. 21 (1): 101–6. doi:10.1093/jac/21.1.101. PMID 3356617.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - The quinolones (Third Edition 2000) By Vincent T. Andriole Chapter I History and overview By Dr. Peter Ball (page 5)
- King DE, Malone R, Lilley SH (2000). "New classification and update on the quinolone antibiotics". American Family Physician. 61 (9): 2741–8. PMID 10821154.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Generics (UK) Ltd v Daiichi Pharmaceutical Co Ltd". Reports of Patent, Design and Trade Mark Cases. 126: 102. 2009. doi:10.1093/rpc/rcn037.
- "Substituted benzo(ij)quinolizine-2-carboxylic acids and derivatives thereof - Patent 3896131". Freepatentsonline.com. Retrieved 2010-04-04.
- Takahashi H, Hayakawa I, Akimoto T (2003). "". Yakushigaku Zasshi (in Japanese). 38 (2): 161–79. PMID 15143768.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sirbat D, Saudax E, Hurault de Ligny B, Hachet E, Abellan P, George JL (1983). "". Bulletin Des Sociétés D'ophtalmologie De France (in French). 83 (8–9): 1019–21. PMID 6378414.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hurault de Ligny B, Sirbat D, Kessler M, Trechot P, Chanliau J (1984). "". Thérapie (in French). 39 (5): 595–600. PMID 6506018.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ball P (2000). "Quinolone generations: natural history or natural selection?". The Journal of Antimicrobial Chemotherapy. 46 (Suppl T1): 17–24. PMID 10997595.
{{cite journal}}: Unknown parameter|month=ignored (help) - Dubois A, Janbon C, Pignodel C, Marty-Double C (1983). "". Gastroentérologie Clinique et Biologique (in French). 7 (2): 217–8. PMID 6840466.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Pinzani V, Gennaro G, Petit P, Blayac JP (1992). "". Thérapie (in French). 47 (5): 440. PMID 1299991.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Marsepoil T, Blin F, Lo JM, Horel D'Ancona F, Sebbah JL (1985). "". Presse Médicale (in French). 14 (32): 1712. PMID 2932732.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Fluoroquinolones- A Review– Dr.T R RAMANUJAM.M.D". Medindia.net. Retrieved 2010-04-04.
- Karbiwnyk CM, Hibbard LE, Lee RH; et al. (April 27–28, 2005). Confirmation of Oxolinic Acid and Flumequine Residues in Shrimp by Liquid Chromatography with Tandem Mass Spectrometry Detection. FDA Science.
{{cite conference}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Residue found in Catfish/Basa, Shrimp, salmon, trout http://www.fda.gov/downloads/Food/GuidanceComplianceRegulatoryInformation/ComplianceEnforcement/ucm073192.pdf
- "FAO/WHO Expert Committee on Food Additives, Geneva, Switzerland". Fda.gov. 2009-10-28. Retrieved 2010-04-04.
- "FDA Veterinarian Newsletter, Volume XXII, No. V, 2007" (PDF). Retrieved 2010-04-04.
- "Codex Committee on Veterinary Drug Residues Acts on Several Documents at 17th Session". Fda.gov. 2009-10-28. Retrieved 2010-04-04.
- WHO Drug Information Vol. 2, No. 3, 1988
- Drlica K, Zhao X (1997). "DNA gyrase, topoisomerase IV, and the 4-quinolones". Microbiology and Molecular Biology Reviews. 61 (3): 377–92. PMC 232616. PMID 9293187.
{{cite journal}}: Unknown parameter|month=ignored (help) - Robinson MJ, Martin BA, Gootz TD, McGuirk PR, Osheroff N (1992). "Effects of novel fluoroquinolones on the catalytic activities of eukaryotic topoisomerase II: Influence of the C-8 fluorine group". Antimicrobial Agents and Chemotherapy. 36 (4): 751–6. PMC 189387. PMID 1323952.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sissi C, Palumbo M (2003). "The quinolone family: from antibacterial to anticancer agents". Current Medicinal Chemistry. 3 (6): 439–50. doi:10.2174/1568011033482279. PMID 14529452.
The present review focuses on the structural modifications responsible for the transformation of an antibacterial into an anticancer agent. Indeed, a distinctive feature of drugs based on the quinolone structure is their remarkable ability to target different type II topoisomerase enzymes. In particular, some congeners of this drug family display high activity not only against bacterial topoisomerases but also against eukaryotic topoisomerases and are toxic to cultured mammalian cells and in vivo tumor models
{{cite journal}}: Unknown parameter|month=ignored (help) - Hussy P, Maass G, Tümmler B, Grosse F, Schomburg U (1986). "Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts". Antimicrobial Agents and Chemotherapy. 29 (6): 1073–8. PMC 180502. PMID 3015015.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Hosomi J, Maeda A, Oomori Y, Irikura T, Yokota T (1988). "Mutagenicity of Norfloxacin and AM-833 in Bacteria and Mammalian Cells". Reviews of Infectious Diseases. 10 (Supplement 1): S148–9. JSTOR 4454399.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Forsgren A, Bredberg A, Pardee AB, Schlossman SF, Tedder TF (1987). "Effects of ciprofloxacin on eucaryotic pyrimidine nucleotide biosynthesis and cell growth". Antimicrobial Agents and Chemotherapy. 31 (5): 774–9. PMC 174831. PMID 3606077.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Gootz TD, Barrett JF, Sutcliffe JA (1990). "Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems". Antimicrobial Agents and Chemotherapy. 34 (1): 8–12. PMC 171510. PMID 2158274.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Lawrence JW, Darkin-Rattray S, Xie F, Neims AH, Rowe TC (1993). "4-Quinolones cause a selective loss of mitochondrial DNA from mouse L1210 leukemia cells". Journal of Cellular Biochemistry. 51 (2): 165–74. doi:10.1002/jcb.240510208. PMID 8440750.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Elsea SH, Osheroff N, Nitiss JL (1992). "Cytotoxicity of quinolones toward eukaryotic cells. Identification of topoisomerase II as the primary cellular target for the quinolone CP-115,953 in yeast". The Journal of Biological Chemistry. 267 (19): 13150–3. PMID 1320012.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Suto MJ, Domagala JM, Roland GE, Mailloux GB, Cohen MA (1992). "Fluoroquinolones: relationships between structural variations, mammalian cell cytotoxicity, and antimicrobial activity". Journal of Medicinal Chemistry. 35 (25): 4745–50. doi:10.1021/jm00103a013. PMID 1469702.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Enzmann H, Wiemann C, Ahr HJ, Schlüter G (1999). "Damage to mitochondrial DNA induced by the quinolone Bay y 3118 in embryonic turkey liver". Mutation Research. 425 (2): 213–24. doi:10.1016/S0027-5107(99)00044-5. PMID 10216214.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Kashida Y, Sasaki YF, Ohsawa K; et al. (2002). "Mechanistic study on flumequine hepatocarcinogenicity focusing on DNA damage in mice". Toxicological Sciences. 69 (2): 317–21. doi:10.1093/toxsci/69.2.317. PMID 12377980.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Thomas A, Tocher J, Edwards DI (1990). "Electrochemical characteristics of five quinolone drugs and their effect on DNA damage and repair in Escherichia coli". The Journal of Antimicrobial Chemotherapy. 25 (5): 733–44. doi:10.1093/jac/25.5.733. PMID 2165050.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Fluoroquinolones and Quinolones". The American Academy of Optometry (British Chapter). Retrieved 29 January 2009.
- Al-Soud, Yaseen A.; Al-Masoudi, Najim A. (2003). "A new class of dihaloquinolones bearing N'-aldehydoglycosylhydrazides, mercapto-1,2,4-triazole, oxadiazoline and a-amino ester precursors: synthesis and antimicrobial activity". Journal of the Brazilian Chemical Society. 14. doi:10.1590/S0103-50532003000500014.
- Daudon M, Protat MF, Réveillaud RJ (1983). "". Annales De Biologie Clinique (in French). 41 (4): 239–49. PMID 6139048.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Rincé C, Daudon M, Moesch C, Rincé M, Leroux-Robert C (1987). "Identification of flumequine in a urinary calculus". Journal of Clinical Chemistry and Clinical Biochemistry. 25 (5): 313–4. PMID 3612030.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Reveillaud RJ, Daudon M (1983). "". Presse Médicale (in French). 12 (38): 2389–92. PMID 6138768.
{{cite journal}}: Unknown parameter|month=ignored (help) - Allergy to quinolones Eight cases of quinolone allergy F. F. Arboit 1 , JC Bessot 2 , F. Arboit 1, JC Bessot 2, F. De Blay 2 , A. De Blay 2, A. Dietemann 2 , C. Dietemann 2, C. Charpentier 2 and G. Carpenter 2 and G. Pauli 2 , Pauli 2, 1 Hôpital Belle-Ile, Service de Pneumologie, 57045 METZ Cedex, France 1 Belle-Isle Hospital, Department of Pneumology, 57045 METZ Cedex, France 2 Service de Pneumologie, Pavillon Laennec, Hôpitaux Universitaires de Strasbourg, BP 426, 67091 STRASBOURG Cedex, France 2 Service de Pneumologie, Pavillon Laennec, Hôpitaux Universitaires de Strasbourg, BP 426, 67091 Strasbourg Cedex, France
- Adverse Reaction Newsletter 1996:1 WHO collaborating centre for international drug monitoring
- Christ W (1990). "Central nervous system toxicity of quinolones: human and animal findings". The Journal of Antimicrobial Chemotherapy. 26 (Suppl B): 219–25. PMID 2124211.
{{cite journal}}: Unknown parameter|month=ignored (help) - Defoin JF, Debonne T, Rambourg MO; et al. (1990). "". Journal De Toxicologie Clinique et Expérimentale (in French). 10 (7–8): 469–72. PMID 2135062.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Rampa S, Caroli F (1991). "". L'Encéphale (in French). 17 (6): 511–4. PMID 1666873.
- Vermeersch G, Filali A, Marko J, Catteau JP, Couture A (1990). "". Journal De Pharmacie De Belgique (in French). 45 (5): 299–305. PMID 1964964.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Revuz J, Pouget F (1983). "". Annales De Dermatologie et De Vénéréologie (in French). 110 (9): 765. PMID 6660786.
- Bailey RR, Natale R, Linton AL (1972). "Nalidixic acid arthralgia". Canadian Medical Association Journal. 107 (7): 604 passim. PMC 1940945. PMID 4541768.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Bailey RR, Kirk JA, Peddie BA (1983). "Norfloxacin-induced rheumatic disease". The New Zealand Medical Journal. 96 (736): 590. PMID 6223241.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Szarfman A, Chen M, Blum MD (1995). "More on fluoroquinolone antibiotics and tendon rupture". The New England Journal of Medicine. 332 (3): 193. doi:10.1056/NEJM199501193320319. PMID 7800023.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - "Petition to Require a Warning on All Fluoroquinolone Antibiotics (HRG Publication #1399)". Public Citizen. August 1, 1996. Retrieved on December 27, 2008.
- "Reports of adverse events with fluoroquinolones" (Doc). FDA Medical Bulletin. 26 (3). October 1996.
- ^ "Madigan, Public Citizen, petition FDA for "Black Box" warning regarding potential adverse effects of certain popular antibiotics" (Press release). Office of the Illinois Attorney General. August 29, 2006. Retrieved 2008-12-27. Full text of the 2005 petition and FDA response available from the Fluoroquinolone Toxicity Research Foundation, a U.S. consumer advocacy group.
- "Public Citizen Petitions the FDA to Include a Black Box Warning on Fluoroquinolone Antibiotics (HRG Publication #1781)". Public Citizen. August 29, 2006. Retrieved 2008-12-27.
- "Public Citizen v. Food and Drug Administration (FDA) (Fluoroquinolone)". Public Citizen. January 3, 2008. Retrieved 2008-12-27.
- Ravn, Karen (August 18, 2008). "Behind the FDA's 'black box' warnings". Los Angeles Times. Retrieved 2008-12-27.
- "FDA Requests Boxed Warnings on Fluoroquinolone Antimicrobial Drugs" (Press release). U.S. Food and Drug Administration. 2008-07-08. Retrieved 2008-10-11.
- The complete labeling history of each drug is available from Drugs@FDA. Medication Guides are available from the FDA's MedWatch system.
- MacCarthy, Paul (October 22, 2008). "Important Change in the Avelox (moxifloxacin hydrochloride) and Cipro (ciprofloxacin) Complete Prescribing Information – Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture" (PDF). Bayer HealthCare Pharmaceuticals. Retrieved 2008-12-27.
- Rosenthal, Norman (November 2008). "Important Change in the LEVAQUIN (Ievofloxacin) Complete Prescribing Information -Addition of Boxed Warning and Medication Guide Regarding Tendinitis and Tendon Rupture" (PDF). Ortho-McNeil Janssen Scientific Affairs, LLC. Retrieved 2008-12-27.
- Lacarelle B, Blin O, Audebert C; et al. (1994). "The quinolone, flumequine, has no effect on theophylline pharmacokinetics". European Journal of Clinical Pharmacology. 46 (5): 477–8. PMID 7957547.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - "Safety (MSDS) data for flumequine". Msds.chem.ox.ac.uk. 2006-07-24. Retrieved 2010-04-04.
- "Flumequine(antiniotic antimicrobial agents) Manufacturers & Suppliers". 88chem.com. Retrieved 2010-04-04.
- Flumequine First draft prepared by Philip G. Francis Russet House, Shere Road West Horsley, Surrey, England and Dr. Robert J. Wells Australian Government Analytical Laboratories Pymble, Australia http://www.fao.org/docrep/W8338E/w8338e0a.htm
| Antibacterials that inhibit nucleic acid (J01E, J01M) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antifolates (inhibit bacterial purine metabolism, thereby inhibiting DNA and RNA synthesis) |
| ||||||||||||||||
| Quinolones (inhibit bacterial topoisomerase and/or DNA gyrase, thereby inhibiting DNA replication) |
| ||||||||||||||||
| Anaerobic DNA inhibitors |
| ||||||||||||||||
| RNA synthesis |
| ||||||||||||||||
| |||||||||||||||||