This is an old revision of this page, as edited by Beetstra (talk | contribs) at 11:00, 11 November 2011 (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank').). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 11:00, 11 November 2011 by Beetstra (talk | contribs) (Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank').)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff) Pharmaceutical compound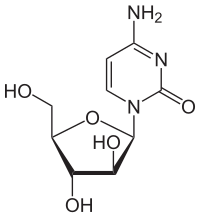 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682222 |
| Pregnancy category |
|
| Routes of administration | Injectable (intravenous injection or infusion, intrathecal, or subcutaneously) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 20% oral |
| Protein binding | 13% |
| Metabolism | Liver |
| Elimination half-life | biphasic: 10 min, 1-3 hr |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.188 |
| Chemical and physical data | |
| Formula | C9H13N3O5 |
| Molar mass | 243.217 g/mol g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Cytarabine, or cytosine arabinoside, is a chemotherapy agent used mainly in the treatment of cancers of white blood cells such as acute myeloid leukemia (AML) and non-Hodgkin lymphoma. It is also known as Ara-C (Arabinofuranosyl Cytidine). It destroys cancer cells by interfering with DNA synthesis.
It is called cytosine arabinoside because it combines a cytosine base with an arabinose sugar. Cytosine normally combines with a different sugar, deoxyribose, to form deoxycytidine, a component of DNA. Certain sponges, where it was originally found, use arabinoside sugars to form a different compound (not part of DNA). Cytosine arabinoside is similar enough to human cytosine deoxyribose (deoxycytidine) to be incorporated into human DNA, but different enough that it kills the cell. This mechanism is used to kill cancer cells. Cytarabine is the first of a series of cancer drugs that altered the sugar component of nucleosides. Other cancer drugs modify the base.
History
Cytarabine was first synthesized in 1959 by Richard Walwick, Walden Roberts, and Charles Dekker at the University of California, Berkeley.
It was approved by the United States Food and Drug Administration in June 1969, and was initially marketed in the US by Upjohn under the trade name Cytosar-U.
Pharmacology
Mechanism
Cytosine arabinoside interferes with the synthesis of DNA. It is an antimetabolic agent with the chemical name of 1β-arabinofuranosylcytosine. Its mode of action is due to its rapid conversion into cytosine arabinoside triphosphate, which damages DNA when the cell cycle holds in the S phase (synthesis of DNA). Rapidly dividing cells, which require DNA replication for mitosis, are therefore most affected. Cytosine arabinoside also inhibits both DNA and RNA polymerases and nucleotide reductase enzymes needed for DNA synthesis.
When used as an antiviral, cytarabine functions by inhibiting deoxycytidine use.
Cytarabine is rapidly deaminated in the body into the inactive uracil derivative and therefore is often given by continuous intravenous infusion.
Pharmacokinetics
Orally, less than 20% of a dose of cytarabine is absorbed from the gastrointestinal tract and is ineffective by this route. Subcutaneously or intramuscularly, tritium-labelled cytarabine produces peak plasma concentrations of radioactivity within 20 to 60 minutes which are considerably lower than those attained after intravenous administration. Continuous intravenous infusions produce relatively constant plasma levels in 8 to 24 hours.
Intravenous doses of cytarabine exhibit a biphasic elimination, with an initial distribution half-life of about ten minutes during which time a major portion of the drug is metabolised in the liver to the inactive metabolite uracil arabinoside. The secondary elimination half-life is longer, approximately one to three hours. Metabolism also occurs in the kidneys, gastrointestinal mucosa, granulocytes and other tissues.
Cytarabine is mainly excreted via the kidney with 70 to 80% of a dose administered by any route appearing in the urine within 24 hours; approximately 90% as the metabolite and 10% as unchanged drug.
Clinical uses
Cytarabine is mainly used in the treatment of acute myeloid leukaemia, acute lymphocytic leukaemia (ALL) and in lymphomas, where it is the backbone of induction chemotherapy.
Cytarabine also possesses antiviral activity, and it has been used for the treatment of generalised herpesvirus infection. However, cytarabine is not very selective in this setting and causes bone marrow suppression and other severe side effects, so it is used mainly for the chemotherapy of hematologic cancers.
Cytarabine is also used in the study of the nervous system to control the proliferation of glial cells in cultures, the amount of glial cells having an important impact on neurons.
Side effects
One of the unique toxicities of cytarabine is cerebellar toxicity when given in high doses.
Possible infection resulting from granulocytopenia and other impaired body defences, and hemorrhage secondary to thrombocytopenia
Toxicity: Leukopenia, Thrombocytopenia, anemia, GI disturbances, stomatitis, conjunctivitis, pneumonitis, fever, and dermatitis, Palmar-Plantar Erythrodysesthesia.
Rarely, myelopathy has been reported after high dose or frequent intrathecal Ara-C administration.
Brand names
- Cytosar-U
- Tarabine PFS (Pfizer)
- Depocyt (longer-lasting liposomal formulation)
- AraC
References
- "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- Wang WS, Tzeng CH, Chiou TJ; et al. (1997). "High-dose cytarabine and mitoxantrone as salvage therapy for refractory non-Hodgkin's lymphoma" (). Jpn. J. Clin. Oncol. 27 (3): 154–7. doi:10.1093/jjco/27.3.154. PMID 9255269.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Ogbomo H, Michaelis M, Klassert D, Doerr HW, Cinatl J (2008). "Resistance to cytarabine induces the up-regulation of NKG2D ligands and enhances natural killer cell lysis of leukemic cells". Neoplasia. 10 (12): 1402–10. PMC 2586691. PMID 19048119.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Feist, Patty (April 2005). "A Tale from the Sea to Ara C".
- Sneader, Walter (2005). Drug discovery: a history. New York: Wiley. p. 258. ISBN 0-471-89979-8.
- Perry, Michael J. (2008). The Chemotherapy source book. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 80. ISBN 0-7817-7328-8.
- Lemke, Thomas L.; Williams, David H.; Foye, William O. (2002). Foye's principles of medicinal chemistry. Hagerstwon, MD: Lippincott Williams & Wilkins. p. 963. ISBN 0-683-30737-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Pigneux A, Perreau V, Jourdan E; et al. (2007). "Adding lomustine to idarubicin and cytarabine for induction chemotherapy in older patients with acute myeloid leukemia: the BGMT 95 trial results". Haematologica. 92 (10): 1327–34. doi:10.3324/haematol.11068. PMID 18024370.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Watterson J, Toogood I, Nieder M; et al. (1994). "Excessive spinal cord toxicity from intensive central nervous system-directed therapies". Cancer. 74 (11): 3034–41. doi:10.1002/1097-0142(19941201)74:11<3034::AID-CNCR2820741122>3.0.CO;2-O. PMID 7954266.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- MedlinePlus page on cytarabine
- ADAP drugs page on cytarabine
- BC Cancer network page on cytarabine
- Chembank entry
- Sea to Ara C An essay on the history of cytarabine.
| DNA virus antivirals (primarily J05, also S01AD and D06BB) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baltimore I |
| ||||||||||||||||||||
| Hepatitis B (VII) | |||||||||||||||||||||
| Multiple/general |
| ||||||||||||||||||||
| |||||||||||||||||||||