| |

| |
| Names | |
|---|---|
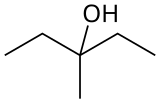
| Preferred IUPAC name 3-Methylpentan-3-ol | |
| Other names
3-Methyl-3-pentanol Diethyl carbinol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.959 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H14O |
| Molar mass | 102.174 g/mol |
| Appearance | colorless liquid |
| Odor | fruity |
| Density | 0.8286 g/cm at 20 °C |
| Melting point | −23.6 °C (−10.5 °F; 249.6 K) |
| Boiling point | 122.4 °C (252.3 °F; 395.5 K) |
| Solubility in water | 45 g/L |
| Solubility | miscible with ethanol, diethyl ether |
| Thermochemistry | |
| Heat capacity (C) | 293.4 J·mol·K (liquid) |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H226, H302 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P264, P270, P280, P301+P312, P303+P361+P353, P330, P370+P378, P403+P235, P501 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 710 mg/kg rat |
| Safety data sheet (SDS) | http://www.sciencelab.com/msds.php?msdsId=9926087 |
| Related compounds | |
| Related compounds | Hexanol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
3-Methyl-3-pentanol (IUPAC name: 3-methylpentan-3-ol) is an organic chemical compound and a tertiary hexanol. It is used in the synthesis of the tranquilizer emylcamate, and has similar sedative and anticonvulsant actions itself.
Synthesis
It can be prepared by reacting ethylmagnesium bromide with methyl acetate in the so-called Grignard reaction using dried diethyl ether or tetrahydrofuran as solvent.

It can be prepared also by reacting ethylmagnesium bromide with butanone in the same conditions already mentioned.
References
- Lide DR (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 3–400, 5–47, 8–106. ISBN 0-8493-0594-2.
- Sittig M (1988). Pharmaceutical manufacturing encyclopedia. Vol. 2 (2 ed.). William Andrew. pp. 555–556. ISBN 978-0-8155-1144-1. Retrieved 2010-01-22.
- Brown B, Schaffarzick RW, Dreisbach RH (October 1955). "Anticonvulsant properties of certain secondary and tertiary alcohols". The Journal of Pharmacology and Experimental Therapeutics. 115 (2): 230–9. PMID 13272171.
This article about an alcohol is a stub. You can help Misplaced Pages by expanding it. |