 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H29FN4O2 |
| Molar mass | 400.498 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
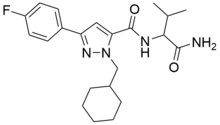
AB-CHFUPYCA (also AB-CHMFUPPYCA) is a compound that was first identified as a component of synthetic cannabis products in Japan in 2015. The name "AB-CHFUPYCA" is an acronym of its systematic name N-(1-Amino-3-methyl-1-oxoButan-2-yl)-1-(CycloHexylmethyl)-3-(4-FlUorophenyl)-1H-PYrazole-5-CarboxAmide. There are two known regioisomers of AB-CHFUPYCA: 3,5-AB-CHMFUPPYCA (pictured) and 5,3-AB-CHMFUPPYCA. The article refers to both 3,5-AB-CHMFUPPYCA and 5,3-AB-CHMFUPPYCA as AB-CHMFUPPYCA isomers, so AB-CHMFUPPYCA and AB-CHFUPYCA are not names for a unique chemical structure.
AB-CHFUPYCA contains some similar structural elements to other synthetic cannabinoids such as 5F-AB-FUPPYCA, JWH-307, JWH-030, JWH-147, AB-PINACA. It may be considered an analog of the traditional pyrazole cannabinoid receptor 1 antagonist rimonabant. The pharmacological properties of AB-CHFUPYCA have not been studied.
See also
- 5F-AB-FUPPYCA
- AM-6545
- TM-38837
- Rimonabant
- JWH-307
- JWH-147
- JWH-030
- 5F-PB22
- 5F-AB-PINACA
- 5F-ADB
- 5F-AMB
- 5F-APINACA
- AB-CHMFUPPYCA
- AB-FUBINACA
- AB-PINACA
- ADB-CHMINACA
- ADB-FUBINACA
- ADB-PINACA
- ADBICA
- APICA
- APINACA
- MDMB-CHMICA
References
- McLaughlin G, Morris N, Kavanagh PV, Power JD, Twamley B, O'Brien J, et al. (September 2016). "The synthesis and characterization of the 'research chemical' N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(cyclohexylmethyl)-3-(4-fluorophenyl)-1H-pyrazole-5-carboxamide (3,5-AB-CHMFUPPYCA) and differentiation from its 5,3-regioisomer" (PDF). Drug Testing and Analysis. 8 (9): 920–9. doi:10.1002/dta.1864. PMID 26360802. S2CID 6300462.
- Uchiyama N, Asakawa K, Kikura-Hanajiri R, Tsutsumi T, Hakamatsuka T (August 2015). "A new pyrazole-carboxamide type synthetic cannabinoid AB-CHFUPYCA identified in illegal products". Forensic Toxicology. 33 (2): 367–373. doi:10.1007/s11419-015-0283-8. S2CID 20718579.
- Franz F, Angerer V, Brandt SD, McLaughlin G, Kavanagh PV, Moosmann B, Auwärter V (February 2017). "In vitro metabolism of the synthetic cannabinoid 3,5-AB-CHMFUPPYCA and its 5,3-regioisomer and investigation of their thermal stability" (PDF). Drug Testing and Analysis. 9 (2): 311–316. doi:10.1002/dta.1950. PMID 26888282. S2CID 205762732.
- "5-fluoro-3,5-AB-PFUPPYCA". Cayman Chemical Company. Retrieved 25 May 2017.
This cannabinoid related article is a stub. You can help Misplaced Pages by expanding it. |