 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
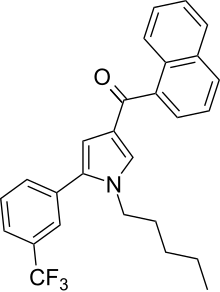
| Formula | C27H24F3NO |
| Molar mass | 435.490 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
JWH-363 (1-Naphthyl{1-pentyl-5--1H-pyrrol-3-yl}methanone) is a synthetic cannabinoid from the naphthoylpyrrole family which acts as an agonist of the CB1 (Ki = 245 ± 5nM) and CB2 (Ki = 71 ± 1nM) receptors, with a moderate (~3.45x) selectivity for the latter. JWH-363 was first synthesized in 2006 by John W. Huffman and colleagues to examine the nature of ligand binding to the CB1 receptor.
Legality
In the United States JWH-363 is not federally scheduled, although some states have passed legislation banning the sale, possession, and manufacture of JWH-363.
In Canada, JWH-363 and other naphthoylpyrrole-based cannabinoids are Schedule II controlled substances under the Controlled Drugs and Substances Act.
In the United Kingdom, JWH-363 and other naphthoylpyrrole-based cannabinoids are considered Class B drugs under the Misuse of Drugs Act 1971.
See also
References
- Huffman JW, Padgett LW, Isherwood ML, Wiley JL, Martin BR (October 2006). "1-Alkyl-2-aryl-4-(1-naphthoyl)pyrroles: new high affinity ligands for the cannabinoid CB1 and CB2 receptors". Bioorganic & Medicinal Chemistry Letters. 16 (20): 5432–5. doi:10.1016/j.bmcl.2006.07.051. PMID 16889960.
- 21 U.S.C. § 812: Schedules of controlled substances
- "The 2020 Florida Statutes". www.leg.state.fl.us. Retrieved 20 August 2021.
- "Arizona Revised Statutes Title 13. Criminal Code § 13-3401". www.azleg.gov. Retrieved 20 August 2021.
- "California Code, Health and Safety Code - HSC § 11357.5". Findlaw.
This cannabinoid related article is a stub. You can help Misplaced Pages by expanding it. |