| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Isotopes of beryllium" – news · newspapers · books · scholar · JSTOR (May 2018) (Learn how and when to remove this message) |
| |||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Be) | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||
Beryllium (4Be) has 11 known isotopes and 3 known isomers, but only one of these isotopes (
Be
) is stable and a primordial nuclide. As such, beryllium is considered a monoisotopic element. It is also a mononuclidic element, because its other isotopes have such short half-lives that none are primordial and their abundance is very low (standard atomic weight is 9.0121831(5)). Beryllium is unique as being the only monoisotopic element with both an even number of protons and an odd number of neutrons. There are 25 other monoisotopic elements but all have odd atomic numbers, and even numbers of neutrons.
Of the 10 radioisotopes of beryllium, the most stable are
Be
with a half-life of 1.387(12) million years and
Be
with a half-life of 53.22(6) d. All other radioisotopes have half-lives under 15 s, most under 30 milliseconds. The least stable isotope is
Be
, with a half-life of 650(130) yoctoseconds.
The 1:1 neutron–proton ratio seen in stable isotopes of many light elements (up to oxygen, and in elements with even atomic number up to calcium) is prevented in beryllium by the extreme instability of
Be
toward alpha decay, which is favored due to the extremely tight binding of
He
nuclei. The half-life for the decay of
Be
is only 81.9(3.7) attoseconds.
Beryllium is prevented from having a stable isotope with 4 protons and 6 neutrons by the very lopsided neutron–proton ratio for such a light element. Nevertheless, this isotope,
Be
, has a half-life of 1.387(12) million years, which indicates unusual stability for a light isotope with such a large neutron/proton imbalance. Other possible beryllium isotopes have even more severe mismatches in neutron and proton number, and thus are even less stable.
Most
Be
in the universe is thought to be formed by cosmic ray nucleosynthesis from cosmic ray spallation in the period between the Big Bang and the formation of the Solar System. The isotopes
Be
, with a half-life of 53.22(6) d, and
Be
are both cosmogenic nuclides because they are made on a recent timescale in the Solar System by spallation, like
C
.
List of isotopes
| Nuclide |
Z | N | Isotopic mass (Da) |
Half-life |
Decay mode |
Daughter isotope |
Spin and parity |
Isotopic abundance | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excitation energy | |||||||||||||||||||
Be |
4 | 1 | 5.03987(215)# | p ? | Li ? |
(1/2+)# | |||||||||||||
Be |
4 | 2 | 6.019726(6) | 5.0(3) zs |
2p | He |
0+ | ||||||||||||
Be |
4 | 3 | 7.01692871(8) | 53.22(6) d | ε | Li |
3/2− | Trace | |||||||||||
Be |
4 | 4 | 8.00530510(4) | 81.9(3.7) as |
α | He |
0+ | ||||||||||||
Be |
16626(3) keV | α | He |
2+ | |||||||||||||||
Be |
4 | 5 | 9.01218306(8) | Stable | 3/2− | 1 | |||||||||||||
Be |
14390.3(1.7) keV | 1.25(10) as |
3/2− | ||||||||||||||||
Be |
4 | 6 | 10.01353469(9) | 1.387(12)×10 y | β | B |
0+ | Trace | |||||||||||
Be |
4 | 7 | 11.02166108(26) | 13.76(7) s | β (96.7(1)%) | B |
1/2+ | ||||||||||||
| βα (3.3(1)%) | Li | ||||||||||||||||||
| βp (0.0013(3)%) | Be | ||||||||||||||||||
Be |
21158(20) keV | 0.93(13) zs |
IT ? | Be ? |
3/2− | ||||||||||||||
Be |
4 | 8 | 12.0269221(20) | 21.46(5) ms | β (99.50(3)%) | B |
0+ | ||||||||||||
| βn (0.50(3)%) | B | ||||||||||||||||||
Be |
2251(1) keV | 233(7) ns | IT | Be |
0+ | ||||||||||||||
Be |
4 | 9 | 13.036135(11) | 1.0(7) zs | n ? | Be ? |
(1/2−) | ||||||||||||
Be |
1500(50) keV | (5/2+) | |||||||||||||||||
Be |
4 | 10 | 14.04289(14) | 4.53(27) ms | βn (86(6)%) | B |
0+ | ||||||||||||
| β (> 9.0(6.3)%) | B | ||||||||||||||||||
| β2n (5(2)%) | B | ||||||||||||||||||
| βt (0.02(1)%) | Be | ||||||||||||||||||
| βα (< 0.004%) | Li | ||||||||||||||||||
Be |
1520(150) keV | (2+) | |||||||||||||||||
Be |
4 | 11 | 15.05349(18) | 790(270) ys | n | Be |
(5/2+) | ||||||||||||
Be |
4 | 12 | 16.06167(18) | 650(130) ys |
2n | Be |
0+ | ||||||||||||
| This table header & footer: | |||||||||||||||||||
- Be – Excited nuclear isomer.
- ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding last digits.
- # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).
-
Modes of decay:
EC: Electron capture IT: Isomeric transition n: Neutron emission p: Proton emission - Bold symbol as daughter – Daughter product is stable.
- ( ) spin value – Indicates spin with weak assignment arguments.
- This isotope has not yet been observed; given data is inferred or estimated from periodic trends.
- ^ Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide.
- Produced in Big Bang nucleosynthesis, but not primordial, as it all quickly decayed to Li
- ^ cosmogenic nuclide
- Intermediate product of triple alpha process in stellar nucleosynthesis as part of the path producing C
- Also often considered spontaneous fission, as
Be
splits into two equal
He
nuclei - Has 1 halo neutron
- Has 4 halo neutrons
Beryllium-7
Beryllium-7 is an isotope with a half-life of 53.3 days that is generated naturally as a cosmogenic nuclide. The rate at which the short-lived
Be
is transferred from the air to the ground is controlled in part by the weather.
Be
decay in the Sun is one of the sources of solar neutrinos, and the first type ever detected using the Homestake experiment. Presence of
Be
in sediments is often used to establish that they are fresh, i.e. less than about 3–4 months in age, or about two half-lives of
Be
.
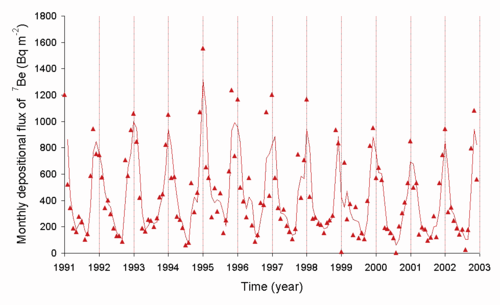
Be
from the air to the ground in Japan
Beryllium-10
Main article: Beryllium-10
Beryllium-10 has a half-life of 1.39×10 y, and decays by beta decay to stable boron-10 with a maximum energy of 556.2 keV. It is formed in the Earth's atmosphere mainly by cosmic ray spallation of nitrogen and oxygen. Be and its daughter product have been used to examine soil erosion, soil formation from regolith, the development of lateritic soils and the age of ice cores. Be is a significant isotope used as a proxy data measure for cosmogenic nuclides to characterize solar and extra-solar attributes of the past from terrestrial samples.
Decay chains
Most isotopes of beryllium within the proton/neutron drip lines decay via beta decay and/or a combination of beta decay and alpha decay or neutron emission. However,
Be
decays only via electron capture, a phenomenon to which its unusually long half-life may be attributed. Notably, its half-life can be artificially lowered by 0.83% via endohedral enclosure (Be@C60). Also anomalous is
Be
, which decays via alpha decay to
He
. This alpha decay is often considered fission, which would be able to account for its extremely short half-life.
Notes
- ^ Note that NUBASE2020 uses the tropical year to convert between years and other units of time, not the Gregorian year. The relationship between years and other time units in NUBASE2020 is as follows: 1 y = 365.2422 d = 31 556 926 s
References
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- "Standard Atomic Weights: Beryllium". CIAAW. 2013.
- Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ Mishra, Ritesh Kumar; Marhas, Kuljeet Kaur (2019-03-25). "Meteoritic evidence of a late superflare as source of 7 Be in the early Solar System". Nature Astronomy. 3 (6): 498–505. Bibcode:2019NatAs...3..498M. doi:10.1038/s41550-019-0716-0. ISSN 2397-3366. S2CID 126552874.
- Wang, Meng; Huang, W.J.; Kondev, F.G.; Audi, G.; Naimi, S. (2021). "The AME 2020 atomic mass evaluation (II). Tables, graphs and references*". Chinese Physics C. 45 (3): 030003. doi:10.1088/1674-1137/abddaf.
- ^ Yamamoto, Masayoshi; Sakaguchi, Aya; Sasaki, Keiichi; Hirose, Katsumi; Igarashi, Yasuhito; Kim, Chang Kyu (January 2006). "Seasonal and spatial variation of atmospheric 210Pb and 7Be deposition: features of the Japan Sea side of Japan". Journal of Environmental Radioactivity. 86 (1): 110–131. doi:10.1016/j.jenvrad.2005.08.001. PMID 16181712.
- G. Korschinek; A. Bergmaier; T. Faestermann; U. C. Gerstmann (2010). "A new value for the half-life of Be by Heavy-Ion Elastic Recoil Detection and liquid scintillation counting". Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 268 (2): 187–191. Bibcode:2010NIMPB.268..187K. doi:10.1016/j.nimb.2009.09.020.
- J. Chmeleff; F. von Blanckenburg; K. Kossert; D. Jakob (2010). "Determination of the Be half-life by multicollector ICP-MS and liquid scintillation counting". Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 268 (2): 192–199. Bibcode:2010NIMPB.268..192C. doi:10.1016/j.nimb.2009.09.012.
- G.A. Kovaltsov; I.G. Usoskin (2010). "A new 3D numerical model of cosmogenic nuclide Be production in the atmosphere". Earth Planet. Sci. Lett. 291 (1–4): 182–199. Bibcode:2010E&PSL.291..182K. doi:10.1016/j.epsl.2010.01.011.
- J. Beer; K. McCracken; R. von Steiger (2012). Cosmogenic radionuclides: theory and applications in the terrestrial and space environments. Physics of Earth and Space Environments. Vol. 26. Physics of Earth and Space Environments, Springer, Berlin. doi:10.1007/978-3-642-14651-0. ISBN 978-3-642-14650-3. S2CID 55739885.
- S.V. Poluianov; G.A. Kovaltsov; A.L. Mishev; I.G. Usoskin (2016). "Production of cosmogenic isotopes Be, Be, C, Na, and Cl in the atmosphere: Altitudinal profiles of yield functions". J. Geophys. Res. Atmos. 121 (13): 8125–8136. arXiv:1606.05899. Bibcode:2016JGRD..121.8125P. doi:10.1002/2016JD025034. S2CID 119301845.
- Balco, Greg; Shuster, David L. (2009). "Al-Be–Ne burial dating" (PDF). Earth and Planetary Science Letters. 286 (3–4): 570–575. Bibcode:2009E&PSL.286..570B. doi:10.1016/j.epsl.2009.07.025. Archived from the original (PDF) on 2015-09-23. Retrieved 2012-12-10.
- Paleari, Chiara I.; F. Mekhaldi; F. Adolphi; M. Christl; C. Vockenhuber; P. Gautschi; J. Beer; N. Brehm; T. Erhardt; H.-A. Synal; L. Wacker; F. Wilhelms; R. Muscheler (2022). "Cosmogenic radionuclides reveal an extreme solar particle storm near a solar minimum 9125 years BP". Nat. Commun. 13 (214): 214. Bibcode:2022NatCo..13..214P. doi:10.1038/s41467-021-27891-4. PMC 8752676. PMID 35017519.
- Ohtsuki, T.; Yuki, H.; Muto, M.; Kasagi, J.; Ohno, K. (9 September 2004). "Enhanced Electron-Capture Decay Rate of 7Be Encapsulated in C60 Cages". Physical Review Letters. 93 (11): 112501. Bibcode:2004PhRvL..93k2501O. doi:10.1103/PhysRevLett.93.112501. PMID 15447332. Retrieved 23 February 2022.
