| |
| Names | |
|---|---|
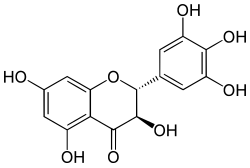
| IUPAC name (2R,3R)-3,3′,4′,5,5′,7-Hexahydroxyflavan-4-one | |
| Systematic IUPAC name (2R,3R)-3,5,7-Trihydroxy-2-(3,4,5-trihydroxy)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names Dihydromyricetin, Ampeloptin,(+)-Ampelopsin,(+)-Dihydromyricetin | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H12O8 |
| Molar mass | 320.253 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ampelopsin, also known as dihydromyricetin and DHM, when used as an effective ingredient in supplements and other tonics, is a flavanonol, a type of flavonoid. It is extracted from the Japanese raisin tree and found in Ampelopsis species japonica, megalophylla, and grossedentata; Cercidiphyllum japonicum; Hovenia dulcis; Rhododendron cinnabarinum; some Pinus species; and some Cedrus species, as well as in Salix sachalinensis.
Hovenia dulcis has been used in traditional Japanese, Chinese, and Korean medicines to treat fever, parasitic infection, as a laxative, and a treatment of liver diseases, and as a hangover treatment. Methods have been developed to extract ampelopsin on a larger scale, and laboratory research has been conducted with the compound to see if it might be useful as a drug in any of the conditions for which the parent plant has been traditionally used.
Research
Research suggests that DHM protects against DOX-induced cardiotoxicity by inhibiting NLRP3 inflammasome activation via stimulation of the SIRT1 pathway.
In a trial of 60 patients with "nonalcoholic fatty liver disease," dihydromyricetin improved glucose and lipid metabolism and yielded potentially beneficial anti-inflammatory effects.
A study of rats demonstrated pharmacological properties of DHM which suggest it would be a therapeutic candidate to treat alcohol use disorders.
Dihydromyricetin shows poor bioavailability which limits its potential medicinal applications.
Additional research is required before claims of human efficacy and application, necessary dosage, and solutions to poor bioavailability, are met with scientific validation.
Applications
Ampelopsin is a versatile compound with a range of applications in health, wellness, and cosmetics, including:
- Anti-Alcohol Intoxication: DHM is widely used in hangover remedies due to its ability to accelerate alcohol breakdown in the liver and mitigate alcohol-induced damage.
- Liver Protection: It helps in protecting the liver from toxins and promoting liver health.
- Antioxidant and Anti-Inflammatory: DHM has strong antioxidant and anti-inflammatory properties, contributing to its potential in preventing and treating chronic diseases.
- Cardiovascular Health: Research indicates that DHM may lower blood pressure and reduce cholesterol levels, benefiting heart health.
- Cosmetic Applications: DHM is used in skincare products for its ability to protect skin from UV-induced damage and aging.
References
- Zhou, Jiaju; Xie, Guirong; Yan, Xinjian (2011-02-21). Encyclopedia of Traditional Chinese Medicines – Molecular Structures, Pharmacological Activities, Natural Sources and Applications: Vol. 1: Isolated Compounds A-C. Springer Science & Business Media. p. 123. ISBN 978-3-642-16735-5.
- Tahara S (June 2007). "A journey of twenty-five years through the ecological biochemistry of flavonoids". Biosci Biotechnol Biochem. 71 (6): 1387–404. doi:10.1271/bbb.70028. PMID 17587669. S2CID 35670587.
- ^ Hyun TK, Eom SH, Yu CY, Roitsch T (July 2010). "Hovenia dulcis--an Asian traditional herb". Planta Med. 76 (10): 943–9. doi:10.1055/s-0030-1249776. PMID 20379955.
- Christidi E, Brunham LR (April 2021). "Regulated cell death pathways in doxorubicin-induced cardiotoxicity". Cell Death Dis. 12 (4): 339. doi:10.1038/s41419-021-03614-x. PMC 8017015. PMID 33795647.
- Chen S, Zhao X, Wan J, Ran L, Qin Y, Wang X, Gao Y, Shu F, Zhang Y, Liu P, Zhang Q, Zhu J, Mi M (September 2015). "Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial". Pharmacol Res. 99: 74–81. doi:10.1016/j.phrs.2015.05.009. PMID 26032587.
- Shen Y, Lindemeyer AK, Gonzalez C, Shao XM, Spigelman I, Olsen RW, Liang J (January 2012). "Dihydromyricetin as a novel anti-alcohol intoxication medication". J Neurosci. 32 (1): 390–401. doi:10.1523/JNEUROSCI.4639-11.2012. PMC 3292407. PMID 22219299.
- Li H, Li Q, Liu Z, Yang K, Chen Z, Cheng Q, Wu L (2017). "The Versatile Effects of Dihydromyricetin in Health". Evid Based Complement Alternat Med. 2017: 1053617. doi:10.1155/2017/1053617. PMC 5602609. PMID 28947908.
- "Dihydromyricetin". Stanford Chemicals. Retrieved July 7, 2024.
- Chen, Jingnan; Wang, Xitong (2021). "Molecular mechanisms and therapeutic implications of dihydromyricetin in liver disease". Biomedicine & Pharmacotherapy. 142. doi:10.1016/j.biopha.2021.111927. PMID 34339914.
- Wen, Chaoyu; Zhang, Fan (2023). "Dihydromyricetin alleviates intestinal inflammation by changing intestinal microbial metabolites and inhibiting the expression of the MyD88/NF-κB signaling pathway". Animal Research and One Health. 1 (2): 219–232. doi:10.1002/aro2.21.
- Zhang, S; Fan, L (2022). "Dihydromyricetin ameliorates osteogenic differentiation of human aortic valve interstitial cells by targeting c-KIT/interleukin-6 signaling pathway". Frontiers in Pharmacology. 13. doi:10.3389/fphar.2022.932092. PMC 9393384. PMID 36003494.
- EP patent 2356980A2
| Flavanonols and their glycosides | |
|---|---|
| 3-Hydroxyflavanones: |
|
| O-methylated flavanonols | |
| dihydroflavonol 3-O-glycosides | |
| Glycosides |
|
| Acetylated glycosides | |