| |

| |
| Names | |
|---|---|
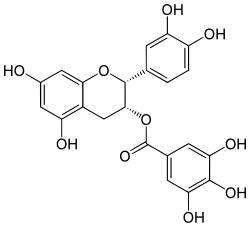
| IUPAC name (2R,3R)-3′,4′,5,7-Tetrahydroxyflavan-3-yl 3,4,5-trihydroxybenzoate | |
| Systematic IUPAC name (2R,3R)-2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate | |
| Other names
ECG Epicatechin 3-gallate (−)-Epicatechin-3-O-gallate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.116.252 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C22H18O10 |
| Molar mass | 442.37 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Epicatechin gallate (ECG) is a flavan-3-ol, a type of flavonoid, present in green tea. It is also reported in buckwheat and in grape.
The tea component epicatechin gallate is being researched because in vitro experiments showed it can reverse methicillin resistance in bacteria such as Staphylococcus aureus. Nevertheless, the compound is significantly degraded by steeping in boiling water, unlike related catechins.
Epicatechin, as well as many other flavonoids, has been found to act as a non-selective antagonist of the opioid receptors, albeit with somewhat low affinity.
References
- ^ Shiota, S; Shimizu, M; Mizushima, T; Ito, H; Hatano, T; Yoshida, T; Tsuchiya, T (1999). "Marked reduction in the minimum inhibitory concentration (MIC) of beta-lactams in methicillin-resistant Staphylococcus aureus produced by epicatechin gallate, an ingredient of green tea (Camellia sinensis)". Biological & Pharmaceutical Bulletin. 22 (12): 1388–90. doi:10.1248/bpb.22.1388. PMID 10746177.
- Danila, Ana-Maria; Kotani, Akira; Hakamata, Hideki; Kusu, Fumiyo (2007). "Determination of Rutin, Catechin, Epicatechin, and Epicatechin Gallate in BuckwheatFagopyrum esculentumMoench by Micro-High-Performance Liquid Chromatography with Electrochemical Detection". Journal of Agricultural and Food Chemistry. 55 (4): 1139–43. doi:10.1021/jf062815i. PMID 17253718.
- Souquet, Jean-Marc; Cheynier, Véronique; Brossaud, Franck; Moutounet, Michel (1996). "Polymeric proanthocyanidins from grape skins". Phytochemistry. 43 (2): 509–512. doi:10.1016/0031-9422(96)00301-9.
- Saklar, Sena; Ertas, Erdal; Ozdemir, Ibrahim S.; Karadeniz, Bulent (October 2015). "Effects of different brewing conditions on catechin content and sensory acceptance in Turkish green tea infusions". Journal of Food Science and Technology. 52 (10): 6639–6646. doi:10.1007/s13197-015-1746-y. PMC 4573099. PMID 26396411.
- Katavic PL, Lamb K, Navarro H, Prisinzano TE (August 2007). "Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships". J. Nat. Prod. 70 (8): 1278–82. doi:10.1021/np070194x. PMC 2265593. PMID 17685652.
See also
| Opioid receptor modulators | |||||
|---|---|---|---|---|---|
| μ-opioid (MOR) |
| ||||
| δ-opioid (DOR) |
| ||||
| κ-opioid (KOR) |
| ||||
| Nociceptin (NOP) |
| ||||
| Others |
| ||||
| Flavan-3-ols and their glycosides | |
|---|---|
| Flavan-3-ols | |
| O-methylated flavan-3ols |
|
| Glycosides |
|
| Acetylated | Phylloflavan |
| Gallate esters | |
| Misc. | |
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |