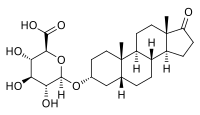
| |
| Names | |
|---|---|
| IUPAC name 17-Oxo-5β-androstan-3α-yl β-D-glucopyranosiduronic acid | |
| Systematic IUPAC name (2S,3S,4S,5R,6R)-6-{phenanthren-7-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid | |
| Other names 5β-Androstan-3α-ol-17-one 3-glucuronide; 3α-Hydroxy-5β-androstan-17-one 3-glucuronide; Etiocholan-3α-ol-17-one 3-glucuronide; 3α-Hydroxyetiocholan-17-one 3-glucuronide; 17-oxoetiocholan-3α-yl β-D-glucopyranosiduronic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C25H38O8 |
| Molar mass | 466.571 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Etiocholanolone glucuronide (ETIO-G) is an endogenous, naturally occurring metabolite of testosterone. It is formed in the liver from etiocholanolone by UDP-glucuronyltransferases. ETIO-G has much higher water solubility than etiocholanolone and is eventually excreted in the urine via the kidneys. Along with androsterone glucuronide, it is one of the major inactive metabolites of testosterone.
See also
References
- ^ "Human Metabolome Database: Showing metabocard for Etiocholanolone glucuronide (HMDB0004484)". Hmdb.ca. Retrieved 2022-04-15.
- ^ S. Bernstein; S. Solomon (6 December 2012). Chemical and Biological Aspects of Steroid Conjugation. Springer Science & Business Media. pp. 328–. ISBN 978-3-642-95177-0.
- David A. Williams; William O. Foye; Thomas L. Lemke (January 2002). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 707–. ISBN 978-0-683-30737-5.
- Christina Wang (28 May 2007). Male Reproductive Function. Springer Science & Business Media. pp. 69–. ISBN 978-0-585-38145-9.
External links
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |