| |
| Names | |
|---|---|
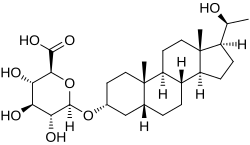
| IUPAC name (20S)-20-Hydroxy-5β-pregnan-3α-yl β-D-glucopyranosiduronic acid | |
| Systematic IUPAC name (2S,3S,4S,5R,6R)-3,4,5-Trihydroxy-6-({(1S,3aS,3bR,5aR,7R,9aS,9bS,11aS)-1--9a,11a-dimethylhexadecahydro-1H-cyclopentaphenanthren-7-yl}oxy)oxane-2-carboxylic acid | |
| Other names Pregnanediol 3α-glucuronide; 5β-Pregnane-3α,20α-diol 3α-glucuronide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C27H44O8 |
| Molar mass | 496.641 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Pregnanediol glucuronide, or 5β-pregnane-3α,20α-diol 3α-glucuronide, is the major metabolite of progesterone and the C3α glucuronide conjugate of pregnanediol (5β-pregnane-3α,20α-diol). Approximately 15 to 30% of a parenteral dose of progesterone is metabolized into pregnanediol glucuronide. While this specific isomer is referred to as pregnanediol glucuronide and is the most major form, there are actually many possible isomers of the metabolite.
References
- ^ Josimovich J (11 November 2013). Gynecologic Endocrinology. Springer Science & Business Media. p. 28. ISBN 978-1-4613-2157-6.
- ^ Etienne-Emile Baulieu; Paul A. Kelly (30 November 1990). Hormones: From Molecules to Disease. Springer Science & Business Media. pp. 401–. ISBN 978-0-412-02791-8.
- Cupps PT (20 February 1991). Reproduction in Domestic Animals. Elsevier. pp. 101–. ISBN 978-0-08-057109-6.
- R. Hobkirk (18 January 2018). Steroid Biochemistry. CRC Press. pp. 23–. ISBN 978-1-351-09380-4.
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |
This biochemistry article is a stub. You can help Misplaced Pages by expanding it. |