| |
| Names | |
|---|---|
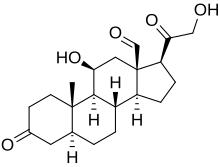
| IUPAC name 11β,21-Dihydroxy-3,20-dioxo-5α-pregnan-18-al | |
| Preferred IUPAC name (1S,3aS,3bS,5aS,9aS,9bS,10S,11aR)-10-Hydroxy-1-(hydroxyacetyl)-9a-methyl-7-oxohexadecahydro-11aH-cyclopentaphenanthrene-11a-carbaldehyde | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C21H30O5 |
| Molar mass | 362.466 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
5α-Dihydroaldosterone is a metabolite of aldosterone that is formed by 5α-reductase. It is a potent antinatriuretic agent similarly to but somewhat different from aldosterone. It is produced in the kidneys.
References
- ^ Azzouni F, Godoy A, Li Y, Mohler J (2012). "The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases". Adv Urol. 2012: 530121. doi:10.1155/2012/530121. PMC 3253436. PMID 22235201.
| Mineralocorticoid receptor modulators | |||||
|---|---|---|---|---|---|
| MRTooltip Mineralocorticoid receptor |
| ||||
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |