 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
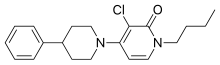
| Formula | C20H25ClN2O |
| Molar mass | 344.88 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
ADX-71149, also known as JNJ-40411813 and JNJ-mGluR2-PAM, is a selective positive allosteric modulator of the mGlu2 receptor. It is being studied by Addex Therapeutics and Janssen Pharmaceuticals for the treatment of schizophrenia. It was also researched by these companies for the treatment of anxious depression (major depressive disorder with anxiety symptoms), but although some efficacy was observed in clinical trials, it was not enough to warrant further development for this indication. As of 2015, ADX-71149 is in phase II clinical trials for schizophrenia.
See also
References
- Cid JM, Tresadern G, Duvey G, Lütjens R, Finn T, Rocher JP, et al. (August 2014). "Discovery of 1-butyl-3-chloro-4-(4-phenyl-1-piperidinyl)-(1H)-pyridone (JNJ-40411813): a novel positive allosteric modulator of the metabotropic glutamate 2 receptor". Journal of Medicinal Chemistry. 57 (15): 6495–512. doi:10.1021/jm500496m. PMID 25032784.
- Lavreysen H, Ahnaou A, Drinkenburg W, Langlois X, Mackie C, Pype S, et al. (February 2015). "Pharmacological and pharmacokinetic properties of JNJ-40411813, a positive allosteric modulator of the mGlu2 receptor". Pharmacology Research & Perspectives. 3 (1): e00096. doi:10.1002/prp2.96. PMC 4317228. PMID 25692015.
- Lavreysen H, Langlois X, Donck LV, Nuñez JM, Pype S, Lütjens R, Megens A (March 2015). "Preclinical evaluation of the antipsychotic potential of the mGlu2-positive allosteric modulator JNJ-40411813". Pharmacology Research & Perspectives. 3 (2): e00097. doi:10.1002/prp2.97. PMC 4324682. PMID 25692027.
- ^ Walker AG, Conn PJ (February 2015). "Group I and group II metabotropic glutamate receptor allosteric modulators as novel potential antipsychotics". Current Opinion in Pharmacology. 20: 40–5. doi:10.1016/j.coph.2014.11.003. PMC 4318747. PMID 25462291.
- Dunlop J, Brandon NJ (February 2015). "Schizophrenia drug discovery and development in an evolving era: are new drug targets fulfilling expectations?". Journal of Psychopharmacology. 29 (2): 230–8. doi:10.1177/0269881114565806. PMID 25586401. S2CID 42542103.
- Addex Therapeutics (7 February 2014). "Addex Reports Top-line Data from ADX71149 Phase 2a Study in Patients with Major Depressive Disorder (MDD) with Significant Anxiety Symptoms". Archived from the original on 25 May 2015. Retrieved 24 May 2015.
External links
- ADX71149 for Schizophrenia - Addex Therapeutics
- ADX71149 for Anxiety - Addex Therapeutics
- ADX 71149 - AdisInsight
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |