| |
| Identifiers | |
|---|---|
| CAS Number | |
| 3D model (JSmol) | |
| ECHA InfoCard | 100.032.026 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | ThS2 |
| Molar mass | 296.17 g/mol |
| Appearance | dark brown crystals |
| Density | 7.3 g/cm, solid |
| Melting point | 1,905 °C (3,461 °F; 2,178 K) |
| Structure | |
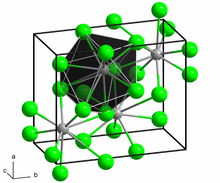
| Crystal structure | PbCl2 type (orthorhombic) |
| Space group | Pnma (No. 62) |
| Formula units (Z) | 4 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Thorium(IV) sulfide (ThS2) is an inorganic chemical compound composed of one thorium atom ionically bonded to two atoms of sulfur. This salt is dark brown and has a melting point of 1905 °C. ThS2 adopts the same orthorhombic lattice structure as PbCl2.
References
- ^ Zachariasen, W. H. (1949-10-01). "Crystal chemical studies of the 5f-series of elements. X. Sulfides and oxysulfides". Acta Crystallographica. 2 (5). International Union of Crystallography (IUCr): 291–296. Bibcode:1949AcCry...2..291Z. doi:10.1107/s0365110x49000758. ISSN 0365-110X.
- Amoretti, G.; Calestani, G.; Giori, D. C. (1984-08-01). "The Refined Structure of ThS2 and the Implications on the Superposition Model Analysis of ThS2: Gd3+ Spin Hamiltonian Parameters". Zeitschrift für Naturforschung A. 39 (8). Walter de Gruyter GmbH: 778–782. doi:10.1515/zna-1984-0809. ISSN 1865-7109.
- Gerward, L.; Staun Olsen, J.; Benedict, U.; Abraham, H. C.; Hulliger, F. (1995). "High-pressure crystal structure of thorium disulfide and diselenide and uranium disulfide". High Pressure Research. 13 (6). Informa UK Limited: 327–333. Bibcode:1995HPR....13..327G. doi:10.1080/08957959508202585. ISSN 0895-7959.
| Thorium compounds | |
|---|---|
| Th(II) | |
| Th(III) | |
| Th(IV) | |
| Sulfides (S) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |