| |
| Names | |
|---|---|
| IUPAC name Zirconium(IV) sulfide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.031.701 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | ZrS2 |
| Molar mass | 155.356 g/mol |
| Appearance | red-brown crystals |
| Density | 3.82 g/cm |
| Melting point | 1,480 °C (2,700 °F; 1,750 K) |
| Solubility in water | insoluble |
| Structure | |
| Crystal structure | Rhombohedral, hP3 |
| Space group | P-3m1, No. 164 |
| Coordination geometry | octahedral |
| Hazards | |
| Safety data sheet (SDS) | MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
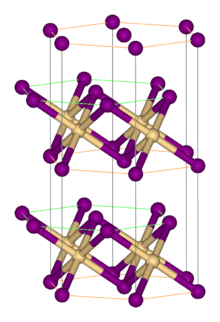
Zirconium(IV) sulfide is the inorganic compound with the formula ZrS2. It is a violet-brown solid. It adopts a layered structure similar to that of cadmium iodide.
Like the closely related titanium disulfide, ZrS2 is prepared by heating sulfur and zirconium metal. It can be purified by vapor transport using iodine.
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 4–96, ISBN 0-8493-0594-2
- Lawrence E. Conroy "Group IV Sulfides" Inorganic Synthesis 1970, XII, 158. doi:10.1002/9780470132432.ch28
| Zirconium compounds | |||||
|---|---|---|---|---|---|
| Zr(II) | |||||
| Zr(III) | |||||
| Zr(IV) |
| ||||
| Sulfides (S) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sulfur compounds | |||||
|---|---|---|---|---|---|
| Sulfides and disulfides |
| ||||
| Sulfur halides | |||||
| Sulfur oxides and oxyhalides |
| ||||
| Sulfur nitrides | |||||
| Thiocyanates | |||||
| Organic compounds | |||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |