| Revision as of 20:09, 28 August 2011 editBogBot (talk | contribs)Bots53,132 edits populated new fields in {{drugbox}} and reordered per bot approval. Report errors and suggestions to User_talk:BogBot← Previous edit | Latest revision as of 06:06, 17 June 2024 edit undoWhywhenwhohow (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers49,181 editsm script-assisted date audit and style fixes per MOS:NUM | ||
| (38 intermediate revisions by 26 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Cholesterol and blood pressure medication}} | |||
| {{Use dmy dates|date=June 2024}} | |||
| {{cs1 config |name-list-style=vanc |display-authors=6}} | |||
| {{Drugbox | {{Drugbox | ||
| | verifiedrevid = |
| verifiedrevid = 424685654 | ||
| | image = |
| image = Atorvastatin.svg | ||
| | |
| width = 250 | ||
| | image2 = Amlodipine.svg | |||
| | width2 = 185 | |||
| <!--Combo data--> | <!--Combo data--> | ||
| Line 12: | Line 17: | ||
| <!--Clinical data--> | <!--Clinical data--> | ||
| | tradename = | | tradename = Caduet, Envacar, others | ||
| | Drugs.com = {{drugs.com|ppa|amlodipine-and-atorvastatin}} | |||
| ⚫ | | |
||
| | |
| DailyMedID = Caduet | ||
| | |
| pregnancy_AU = D | ||
| | pregnancy_US = N | |||
| | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| | pregnancy_category = Contraindicated | |||
| ⚫ | | legal_CA = <!-- |
||
| | legal_AU = S4 | |||
| | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | |||
| ⚫ | | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | ||
| ⚫ | | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | ||
| | legal_US = Rx-only | | legal_US = Rx-only | ||
| | legal_status = |
| legal_status = Rx-only | ||
| | routes_of_administration = Oral | | routes_of_administration = ] | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | CAS_number = | | CAS_number = 858659-02-6 | ||
| | ATC_prefix = C10 | | ATC_prefix = C10 | ||
| | ATC_suffix = BX03 | | ATC_suffix = BX03 | ||
| Line 31: | Line 38: | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 7987512 | | ChemSpiderID = 7987512 | ||
| | KEGG = D08488 | |||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | smiles = Clc1ccccc1C2C(\C(=O)OC)=C(/N\C(=C2\C(=O)OCC)COCCN)C.O=C(O)C(O)C(O)CCn2c(c(c(c2c1ccc(F)cc1)c3ccccc3)C(=O)Nc4ccccc4)C(C)C | |||
| | InChI = 1/C33H35FN2O5.C20H25ClN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40;1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40);5-8,17,23H,4,9-11,22H2,1-3H3/t26-,27-;/m1./s1 | |||
| | InChIKey = PEAJXAZCGDWDGS-CNZCJKERBU | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/C33H35FN2O5.C20H25ClN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40;1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40);5-8,17,23H,4,9-11,22H2,1-3H3/t26-,27-;/m1./s1 | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = PEAJXAZCGDWDGS-CNZCJKERSA-N | |||
| }} | }} | ||
| The drug combination '''atorvastatin/amlodipine''' (trade names '''Caduet''' in the ] and ], and '''Envacar''' elsewhere) is a medication approved by the ] (FDA) for the treatment of ] and ]. It is a ] drug containing the ] ] and the ] ]. It is being marketed by the ] ].<ref>{{cite web|url=http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm202933.htm|format=PDF|title=CADUET (amlodipine besylate/atorvastatin calcium) Tablets|month=February | year=2010|work=NDA 21-540/S-009|publisher=]|accessdate=2010-09-25}}</ref> | |||
| '''Amlodipine/atorvastatin''', sold under the brand name '''Caduet''' among others, is a ] medication for the treatment of ] and ]. It contains a ] and a ].<ref name="Curran 2010 pp. 191–213">{{cite journal | last=Curran | first=Monique P. | title=Amlodipine/Atorvastatin | journal=Drugs | publisher=]| volume=70 | issue=2 | year=2010 | issn=0012-6667 | doi=10.2165/11204420-000000000-00000 | pages=191–213| pmid=20108992 | s2cid=209144335 }}</ref><ref>{{cite journal | vauthors = Devabhaktuni M, Bangalore S | title = Fixed combination of amlodipine and atorvastatin in cardiovascular risk management: patient perspectives | journal = Vascular Health and Risk Management | volume = 5 | issue = 1 | pages = 377–87 | date = 2009 | pmid = 19475775 | pmc = 2686256 | doi = 10.2147/vhrm.s3339 | doi-access = free }}</ref> | |||
| == Society and culture == | |||
| === Brand names === | |||
| Amlodipine/atorvastatin is marketed under the brand name Caduet in the United States, Australia, and Russia, and Envacar in the Philippines.<ref>{{cite web |url=https://www.drugs.com/international/caduet.html |url-status=dead |archive-url=https://web.archive.org/web/20140619222725/http://www.drugs.com/international/caduet.html |archive-date=19 June 2014 |title=Caduet - Drugs.com}}</ref><ref>{{cite web |url=https://www.drugs.com/international/envacar.html |url-status=dead |archive-url=https://web.archive.org/web/20140620180116/http://www.drugs.com/international/envacar.html |archive-date=20 June 2014 |title=Envacar - Drugs.com}}</ref> | |||
| In some countries Caduet is marketed by ] after Upjohn was spun off from Pfizer.<ref>{{cite web | title=Pfizer Completes Transaction to Combine Its Upjohn Business with Mylan | publisher=Pfizer | via=Business Wire | date=16 November 2020 | url=https://www.businesswire.com/news/home/20201116005378/en/ | access-date=17 June 2024}}</ref><ref>{{cite web | title=Caduet | website=Pfizer | url=https://www.pfizer.com/products/product-detail/caduet | access-date=17 June 2024}}</ref><ref>{{cite web | title=Brands | website=Viatris | date=16 November 2020 | url=https://www.viatris.com/en/products/brands | access-date=17 June 2024}}</ref> | |||
| == References == | == References == | ||
| {{reflist}} | {{reflist}} | ||
| {{Lipid modifying agents}} | |||
| == External links == | |||
| {{Portal bar|Medicine}} | |||
| * (caduet.com) | |||
| {{DEFAULTSORT:Amlodipine Atorvastatin}} | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
Latest revision as of 06:06, 17 June 2024
Cholesterol and blood pressure medicationPharmaceutical compound
 | |
 | |
| Combination of | |
|---|---|
| Amlodipine | Calcium channel blocker |
| Atorvastatin | Statin |
| Clinical data | |
| Trade names | Caduet, Envacar, others |
| AHFS/Drugs.com | Professional Drug Facts |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| (verify) | |
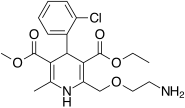
Amlodipine/atorvastatin, sold under the brand name Caduet among others, is a fixed-dose combination medication for the treatment of high cholesterol and high blood pressure. It contains a statin and a calcium channel blocker.
Society and culture
Brand names
Amlodipine/atorvastatin is marketed under the brand name Caduet in the United States, Australia, and Russia, and Envacar in the Philippines.
In some countries Caduet is marketed by Viatris after Upjohn was spun off from Pfizer.
References
- Curran MP (2010). "Amlodipine/Atorvastatin". Drugs. 70 (2). Springer Science+Business Media: 191–213. doi:10.2165/11204420-000000000-00000. ISSN 0012-6667. PMID 20108992. S2CID 209144335.
- Devabhaktuni M, Bangalore S (2009). "Fixed combination of amlodipine and atorvastatin in cardiovascular risk management: patient perspectives". Vascular Health and Risk Management. 5 (1): 377–87. doi:10.2147/vhrm.s3339. PMC 2686256. PMID 19475775.
- "Caduet - Drugs.com". Archived from the original on 19 June 2014.
- "Envacar - Drugs.com". Archived from the original on 20 June 2014.
- "Pfizer Completes Transaction to Combine Its Upjohn Business with Mylan". Pfizer. 16 November 2020. Retrieved 17 June 2024 – via Business Wire.
- "Caduet". Pfizer. Retrieved 17 June 2024.
- "Brands". Viatris. 16 November 2020. Retrieved 17 June 2024.
| Lipid-lowering agents (C10) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI tract |
| ||||||||||||
| Liver |
| ||||||||||||
| Blood vessels |
| ||||||||||||
| Combinations | |||||||||||||
| Other | |||||||||||||
| |||||||||||||
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |