| Revision as of 01:17, 28 February 2016 editOshwah (talk | contribs)Edit filter managers, Autopatrolled, Checkusers, Interface administrators, Oversighters, Administrators496,883 editsm Reverted edits by 2602:304:B35A:F0B0:A512:27:988:B137 (talk) (HG) (3.1.19)← Previous edit | Revision as of 15:48, 10 March 2016 edit undoAlongdwiki (talk | contribs)5 edits updated SMILES to 'N(=O)' and '(=O)'Tag: Visual editNext edit → | ||
| Line 27: | Line 27: | ||
| | RTECS = QW9800000 | | RTECS = QW9800000 | ||
| | Gmelin = 976 | | Gmelin = 976 | ||
| | SMILES = O |
| SMILES = N(=O) | ||
| | |
| SMILES2 = (=O) | ||
| | SMILES2 = | |||
| | StdInChI = 1S/NO2/c2-1-3 | | StdInChI = 1S/NO2/c2-1-3 | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
Revision as of 15:48, 10 March 2016
| |||
 Nitrogen dioxide at −196 °C, 0 °C, 23 °C, 35 °C, and 50 °C | |||
| Names | |||
|---|---|---|---|
| IUPAC name Nitrogen dioxide | |||
| Other names Nitrogen(IV) oxide, Deutoxide of nitrogen | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.030.234 | ||
| EC Number |
| ||
| Gmelin Reference | 976 | ||
| PubChem CID | |||
| RTECS number |
| ||
| UN number | 1067 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | NO 2 | ||
| Molar mass | 46.0055 g mol | ||
| Appearance | Vivid orange gas | ||
| Odor | Chlorine like | ||
| Density | 1.88 g dm | ||
| Melting point | −11.2 °C (11.8 °F; 261.9 K) | ||
| Boiling point | 21.2 °C (70.2 °F; 294.3 K) | ||
| Solubility in water | Hydrolyses | ||
| Solubility | soluble in CCl 4, nitric acid, chloroform | ||
| Vapor pressure | 98.80 kPa (at 20 °C) | ||
| Refractive index (nD) | 1.449 (at 20 °C) | ||
| Structure | |||
| Point group | C2v | ||
| Molecular shape | Bent | ||
| Thermochemistry | |||
| Heat capacity (C) | 37.5 J/mol K | ||
| Std molar entropy (S298) |
240 J·mol·K | ||
| Std enthalpy of formation (ΔfH298) |
+34 kJ·mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Poison, oxidizer | ||
| GHS labelling: | |||
| Pictograms |     
| ||
| Signal word | Danger | ||
| Hazard statements | H270, H314, H330 | ||
| Precautionary statements | P220, P260, P280, P284, P305+P351+P338, P310 | ||
| NFPA 704 (fire diamond) |
 | ||
| Lethal dose or concentration (LD, LC): | |||
| LC50 (median concentration) | 30 ppm (guinea pig, 1 hr) 315 ppm (rabbit, 15 min) 68 ppm (rat, 4 hr) 138 ppm (rat, 30 min) 1000 ppm (mouse, 10 min) | ||
| LCLo (lowest published) | 64 ppm (dog, 8 hr) 64 ppm (monkey, 8 hr) | ||
| NIOSH (US health exposure limits): | |||
| PEL (Permissible) | C 5 ppm (9 mg/m) | ||
| REL (Recommended) | ST 1 ppm (1.8 mg/m) | ||
| IDLH (Immediate danger) | 20 ppm | ||
| Safety data sheet (SDS) | ICSC 0930 | ||
| Related compounds | |||
| Related Nitrogen oxides | Dinitrogen pentoxide Dinitrogen tetroxide | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Nitrogen dioxide is the chemical compound with the formula NO
2. It is one of several nitrogen oxides. NO
2 is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent air pollutant. Nitrogen dioxide is a paramagnetic, bent molecule with C2v point group symmetry.
Molecular properties
Nitrogen dioxide has a molar mass of 46.0055, which makes it heavier than air, whose average molar mass is 28.8.
The bond length between the nitrogen atom and the oxygen atom is 119.7 pm. This bond length is consistent with a bond order between one and two.
Unlike ozone, O3, the ground electronic state of nitrogen dioxide is a doublet state, since nitrogen has one unpaired electron, which decreases the alpha effect compared with nitrite and creates a weak bonding interaction with the oxygen lone pairs. The lone electron in NO
2 also means that this compound is a free radical, so the formula for nitrogen dioxide is often written as ·NO2.
Preparation and reactions
Nitrogen dioxide typically arises via the oxidation of nitric oxide by oxygen in air:
- 2 NO + O
2 → 2 NO
2
In the laboratory, NO
2 can be prepared in a two-step procedure where dehydration of nitric acid produces dinitrogen pentoxide, which subsequently undergoes thermal decomposition:
- 2 HNO
3 → N
2O
5 + H
2O - 2 N
2O
5 → 4 NO
2 + O
2
The thermal decomposition of some metal nitrates also affords NO
2:
- 2 Pb(NO3)2 → 2 PbO + 4 NO
2 + O
2
Alternatively, reduction of concentrated nitric acid by metal (such as copper).
- 4 HNO
3 + Cu → Cu(NO3)2 + 2 NO
2 +2 H2O
Or finally by adding concentrated nitric acid over tin; hydrated tin dioxide is produced as byproduct.
- 4HNO3 + Sn → H2O + H2SnO3 + 4 NO2
Main reactions
Basic thermal properties
NO
2 exists in equilibrium with the colourless gas dinitrogen tetroxide (N
2O
4):
- 2 NO
2 ⇌ N
2O
4
The equilibrium is characterized by ΔH = −57.23 kJ/mol, which is exothermic. NO2 is favored at higher temperatures, while at lower temperatures, dinitrogen tetroxide (N2O4) predominates. Dinitrogen tetroxide (N
2O
4) can be obtained as a white solid with melting point −11.2 °C. NO2 is paramagnetic due to its unpaired electron, while N2O4 is diamagnetic.
The chemistry of nitrogen dioxide has been investigated extensively. At 150 °C, NO
2 decomposes with release of oxygen via an endothermic process (ΔH = 114 kJ/mol):
- 2 NO
2 → 2 NO + O
2
As an oxidizer
As suggested by the weakness of the N–O bond, NO
2 is a good oxidizer. Consequently, it will combust, sometimes explosively, with many compounds, such as hydrocarbons.
Hydrolysis
It hydrolyses to give nitric acid and nitrous acid:
- 2 NO
2/N
2O
4 + H
2O →HNO
2 + HNO
3
This reaction is one step in the Ostwald process for the industrial production of nitric acid from ammonia. Nitric acid decomposes slowly to nitrogen dioxide, which confers the characteristic yellow color of most samples of this acid:
- 4 HNO
3 → 4 NO
2 + 2 H
2O + O
2
Conversion to nitrates
NO
2 is used to generate anhydrous metal nitrates from the oxides:
- MO + 3 NO
2 → M(NO
3)
2 + NO
Conversion to nitrites
Alkyl and metal iodides give the corresponding nitrites:
- 2 CH
3I + 2 NO
2 → 2 CH
3NO
2 + I
2
- TiI
4 + 4 NO
2 → Ti(NO
2)
4 + 2 I
2
Safety and pollution considerations

2) gas converts to the colorless gas dinitrogen tetroxide (N
2O
4) at low temperatures, and converts back to NO
2 at higher temperatures. The bottles in this photograph contain equal amounts of gas at different temperatures.
Nitrogen dioxide is toxic to humans when inhaled. The compound is acrid and easily detectable by smell at low concentrations. However, low concentrations (4 ppm) will anesthetize the nose, thus creating a potential for overexposure. One potential source of exposure is red fuming nitric acid, which spontaneously produces NO
2 above 0 °C. Symptoms of poisoning (lung edema) tend to appear several hours after inhalation of a low but potentially fatal dose.
There is some evidence that long-term exposure to NO
2 at concentrations above 40–100 µg/m may decrease lung function and increase the risk of respiratory symptoms.
Nitrogen dioxide is formed in most combustion processes using air as the oxidant. At elevated temperatures nitrogen combines with oxygen to form nitric oxide:
- O
2 + N
2 → 2 NO
Nitric oxide can be oxidized in air to form nitrogen dioxide. At normal atmospheric concentrations, this is a very slow process.
- 2 NO + O
2 → 2 NO
2
The most prominent sources of NO
2 are internal combustion engines, thermal power stations and, to a lesser extent, pulp mills. Butane gas heaters and stoves are also sources. The excess air required for complete combustion of fuels in these processes introduces nitrogen into the combustion reactions at high temperatures and produces nitrogen oxides (NO
x). Limiting NO
x production demands the precise control of the amount of air used in combustion. In households, kerosene heaters and gas heaters are sources of nitrogen dioxide.
Nitrogen dioxide is also produced by atmospheric nuclear tests, and is responsible for the reddish colour of mushroom clouds.
Nitrogen dioxide is a large scale pollutant, with rural background ground level concentrations in some areas around 30 µg/m, not far below unhealthy levels. Nitrogen dioxide plays a role in atmospheric chemistry, including the formation of tropospheric ozone.
A 2015 study by King's College London concluded that air pollution caused thousands of deaths in London in 2010, specifically identifying NO
2 as the cause of the majority of the deaths. "5,900 deaths were the result of nitrogen dioxide, a pollutant produced by diesel engines"
A 2005 study by researchers at the University of California, San Diego, suggests a link between NO
2 levels and Sudden Infant Death Syndrome.
Nitrogen dioxide is also produced naturally during electrical storms. The term for this process is "atmospheric fixation of nitrogen". The rain produced during such storms is especially good for the garden as it contains trace amounts of fertilizer. (Henry Cavendish 1784, Birkland -Eyde Process 1903, et-al)
 Nitrogen dioxide 2011 – tropospheric column density.
Nitrogen dioxide 2011 – tropospheric column density.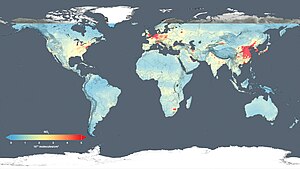 Nitrogen dioxide 2014 – global air quality levels
Nitrogen dioxide 2014 – global air quality levels(released 14 December 2015).
See also
- Dinitrogen tetroxide
- Nitric oxide (NO) – a problematic pollutant that is short lived because it converts to NO
2 in the presence of free oxygen - Nitrite
- Nitrous oxide (N
2O) – "laughing gas", a linear molecule, isoelectronic with CO
2 but with a nonsymmetric arrangement of atoms (NNO) - Nitrogen dioxide poisoning
- Nitryl
References
- "nitrogen dioxide (CHEBI:33101)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. 13 January 2008. Main. Retrieved 4 October 2011.
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. p. 4.79. ISBN 1439855110.
- Mendiara, S. N.; Sagedahl, A.; Perissinotti, L. J. (2001). "An electron paramagnetic resonance study of nitrogen dioxide dissolved in water, carbon tetrachloride and some organic compounds". Applied Magnetic Resonance. 20: 275. doi:10.1007/BF03162326.
- ^ Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 0-618-94690-X.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0454". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Nitrogen dioxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "Nitrogen dioxide". United States Environmental Protection Agency.
- Chemistry of the Elements, N.N. Greenwood, A. Earnshaw, p.455
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Thiemann, Michael; Scheibler, Erich and Wiegand, Karl Wilhelm (2005) "Nitric Acid, Nitrous Acid, and Nitrogen Oxides" in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim doi:10.1002/14356007.a17_293.
- Health Aspects of Air Pollution with Particulate Matter,Ozone and Nitrogen Dioxide (PDF). World Health Organization. 13–15 January 2003. p. 48. Retrieved 2011-11-19.
- Son, Busoon; Wonho Yang; Patrick Breysse; Taewoong Chung; Youngshin Lee (March 2004). "Estimation of occupational and nonoccupational nitrogen dioxide exposure for Korean taxi drivers using a microenvironmental model". Environmental Research. 94 (3): 291–296. doi:10.1016/j.envres.2003.08.004. PMID 15016597. Retrieved 2008-02-25.
- "The Impact of Unvented Gas Heating Appliances on Indoor Nitrogen Dioxide Levels in 'TIGHT' Homes". ashrae.org. Retrieved 2013-04-11.
- Effects of Nuclear Explosions. Nuclearweaponarchive.org. Retrieved on 2010-02-08.
- "Pollution Killing 9,500 Londoners Revives Mayor's Heathrow Plea". Retrieved 2015-07-15.
- "Sids Linked to Nitrogen Dioxide Pollution". Retrieved 2008-02-25.
- Cole, Steve; Gray, Ellen (14 December 2015). "New NASA Satellite Maps Show Human Fingerprint on Global Air Quality". NASA. Retrieved 14 December 2015.
External links
- International Chemical Safety Card 0930
- National Pollutant Inventory – Oxides of nitrogen fact sheet
- NIOSH Pocket Guide to Chemical Hazards
- WHO-Europe reports: Health Aspects of Air Pollution (2003) (PDF) and "Answer to follow-up questions from CAFE (2004) (PDF)
- Nitrogen Dioxide Air Pollution
- Nitrogen dioxide pollution in the world (image)
- A review of the acute and long term impacts of exposure to nitrogen dioxide in the United Kingdom IOM Research Report TM/04/03
| Nitrogen species | |
|---|---|
| Hydrides | |
| Organic | |
| Oxides | |
| Halides | |
| Oxidation states | −3, −2, −1, 0, +1, +2, +3, +4, +5 (a strongly acidic oxide) |

