| |
| Names | |
|---|---|
| IUPAC name Copper(I) sulfide | |
| Other names
Cuprous sulfide Chalcocite Copper glance | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.040.751 |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Cu2S |
| Molar mass | 159.16 g/mol |
| Density | 5.6 g/cm |
| Melting point | 1,130 °C (2,070 °F; 1,400 K) |
| Solubility in water | insoluble |
| Solubility | slightly soluble in HCl; soluble in NH4OH; dissolves in KCN; decomposes in HNO3, H2SO4 |
| Hazards | |
| Flash point | Nonflammable |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | TWA 1 mg/m (as Cu) |
| REL (Recommended) | TWA 1 mg/m (as Cu) |
| IDLH (Immediate danger) | TWA 100 mg/m (as Cu) |
| Related compounds | |
| Other anions | Copper(I) oxide Copper(I) selenide |
| Other cations | Nickel(II) sulfide Copper(II) sulfide Zinc sulfide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Copper(I) sulfide is a copper sulfide, a chemical compound of copper and sulfur. It has the chemical compound Cu2S. It is found in nature as the mineral chalcocite. It has a narrow range of stoichiometry ranging from Cu1.997S to Cu2.000S. Samples are typically black.
Preparation and reactions
Cu2S can be prepared by treating copper with sulfur or H2S. The rate depends on the particle size and temperature. Cu2S reacts with oxygen to form SO2:
- 2 Cu2S + 3 O2 → 2 Cu2O + 2 SO2
The production of copper from chalcocite is a typical process in extracting the metal from ores. Usually, the conversion involves roasting, to give Cu2O and sulfur dioxide:
- Cu2S + O2 → 2 Cu + SO2
Cuprous oxide readily converts to copper metal upon heating.
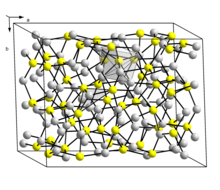
Structure

Stoichiometric
Two forms (a dimorphism) of Cu2S are known. The so-called low temperature monoclinic form ("low-chalcocite") has a complex structure with 96 copper atoms in the unit cell. The hexagonal form, stable above 104 °C, has 24 crystallographically distinct Cu atoms. Its structure has been described as approximating to a hexagonal close packed array of sulfur atoms with Cu atoms in planar 3 coordination. This structure was initially assigned an orthorhombic cell due to the twinning of the sample crystal.
Non-stoichiometric
As illustrated by the mineral djurleite, a cuprous sulfide is also known. With the approximate formula Cu1.96S, this material is non-stoichiometric (range Cu1.934S-Cu1.965S) and has a monoclinic structure with 248 copper and 128 sulfur atoms in the unit cell. Cu2S and Cu1.96S are similar in appearance and hard to distinguish one from another.
Phase transition
The electrical resistivity increases abruptly at the phase transition point around 104 °C, with the precise temperature depending on the stoichiometry.
See also
- Copper sulfide for an overview of all copper sulfide phases
- Copper monosulfide, CuS
- Chalcocite
- Djurleite
- LK-99 - compound evaluated in 2023 for possible superconductivity
References
- Patnaik, Pradyot (2002). Handbook of Inorganic Chemicals. McGraw-Hill, ISBN 0-07-049439-8
- ^ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 1373. ISBN 978-0-08-022057-4.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH).
- Potter, R. W. (1977). "An electrochemical investigation of the system copper-sulfur". Economic Geology. 72 (8): 1524–1542. Bibcode:1977EcGeo..72.1524P. doi:10.2113/gsecongeo.72.8.1524.
- Blachnik R., Müller A. (2000). "The formation of Cu2S from the elements I. Copper used in form of powders". Thermochimica Acta. 361: 31. doi:10.1016/S0040-6031(00)00545-1.
- ^ Wiberg, Egon and Holleman, Arnold Frederick (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- ^ Evans, H. T. (1979). "Djurleite (Cu1.94S) and Low Chalcocite (Cu2S): New Crystal Structure Studies". Science. 203 (4378): 356–8. Bibcode:1979Sci...203..356E. doi:10.1126/science.203.4378.356. PMID 17772445. S2CID 6132717.
- Wells A.F. (1984) Structural Inorganic Chemistry, 5th ed., Oxford Science Publications, ISBN 0-19-855370-6
- Evans H.T. (1981). "Copper coordination in low chalcocite and djurleite and other copper-rich sulfides" (PDF). American Mineralogist. 66 (7–8): 807–818.
- Garisto, Dan (2023-08-16). "LK-99 isn't a superconductor — how science sleuths solved the mystery". Nature. 620 (7975): 705–706. Bibcode:2023Natur.620..705G. doi:10.1038/d41586-023-02585-7. PMID 37587284. S2CID 260955242.
- Jain, Prashant K. "Phase transition of copper (I) sulfide and its implication for purported superconductivity of LK-99." arXiv preprint arXiv:2308.05222 (2023).
| Copper compounds | |
|---|---|
| Cu(0,I) | |
| Cu(I) | |
| Cu(I,II) | |
| Cu(II) | |
| Cu(III) | |
| Cu(IV) | |
| Sulfides (S) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||