 | |
| Clinical data | |
|---|---|
| Other names | PF-06882961 |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
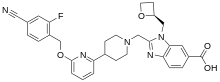
| Formula | C31H30FN5O4 |
| Molar mass | 555.610 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Danuglipron is a small-molecule GLP-1 agonist developed by Pfizer that, in an oral formulation, is under investigation as a therapy for diabetes mellitus. Initial results from a randomized controlled trial indicate that it reduced weight and improved diabetic control. The most commonly reported adverse events were nausea, diarrhea, and vomiting.
See also
References
- Griffith DA, Edmonds DJ, Fortin JP, Kalgutkar AS, Kuzmiski JB, Loria PM, et al. (June 2022). "A Small-Molecule Oral Agonist of the Human Glucagon-like Peptide-1 Receptor". Journal of Medicinal Chemistry. 65 (12): 8208–8226. doi:10.1021/acs.jmedchem.1c01856. PMC 9234956. PMID 35647711.
- Medical Weight Loss
- Saxena AR, Frias JP, Brown LS, Gorman DN, Vasas S, Tsamandouras N, Birnbaum MJ (May 2023). "Efficacy and Safety of Oral Small Molecule Glucagon-Like Peptide 1 Receptor Agonist Danuglipron for Glycemic Control Among Patients With Type 2 Diabetes: A Randomized Clinical Trial". JAMA Network Open. 6 (5): e2314493. doi:10.1001/jamanetworkopen.2023.14493. PMC 10203889. PMID 37213102.
- Constantino, Annika Kim (2023-12-02). "Pfizer's twice-daily weight loss pill joins a long list of obesity drug flops". CNBC. Retrieved 2023-12-03.
This drug article relating to the gastrointestinal system is a stub. You can help Misplaced Pages by expanding it. |