 | |
| Clinical data | |
|---|---|
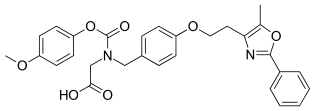
| Other names | 2-phenyl]methyl]amino]acetic acid |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C29H28N2O7 |
| Molar mass | 516.550 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Muraglitazar (proposed tradename Pargluva) is a dual peroxisome proliferator-activated receptor agonist with affinity to PPARα and PPARγ.
The drug had completed phase III clinical trials, however in May 2006 Bristol-Myers Squibb announced that it had discontinued further development.
Data on muraglitazar is relatively sparse due to the brief introduction and subsequent abandonment of this agent. One double-blind randomized clinical trial comparing muraglitazar and pioglitazone found that the effects of the former were favourable in terms of HDL-C increase, decrease in total cholesterol, apolipoprotein B, triglycerides and a greater reduction in HbA1c (p <0.0001 for all comparisons). However, the muraglitazar group had a higher all-cause mortality, greater incidence of edema and heart failure and more weight gain compared to the pioglitazone group. A meta-analysis of the phase II and III clinical trials of muraglitazar revealed that it was associated with a greater incidence of myocardial infarction, stroke, transient ischemic attacks and congestive heart failure (CHF) when compared to placebo or pioglitazone.
By calling attention to adverse events made public through the FDA advisory committee process, Dr Nissen came upon a mechanism to steer FDA from the outside. This mechanism came to fruition with rosiglitazone (Avandia) and led to FDA requiring demonstration of cardiac safety for new drugs to treat type 2 diabetes. This process is described by Dr Robert Misbin in INSULIN-History from an FDA Insider, published June 1, 2020 on Amazon.
References
- Waites CR, Dominick MA, Sanderson TP, Schilling BE (November 2007). "Nonclinical safety evaluation of muraglitazar, a novel PPARalpha/gamma agonist". Toxicological Sciences. 100 (1): 248–58. doi:10.1093/toxsci/kfm193. PMID 17675651.
- ^ Kendall DM, Rubin CJ, Mohideen P, Ledeine JM, Belder R, Gross J, et al. (May 2006). "Improvement of glycemic control, triglycerides, and HDL cholesterol levels with muraglitazar, a dual (alpha/gamma) peroxisome proliferator-activated receptor activator, in patients with type 2 diabetes inadequately controlled with metformin monotherapy: A double-blind, randomized, pioglitazone-comparative study" (PDF). Diabetes Care. 29 (5): 1016–23. doi:10.2337/diacare.2951016. PMID 16644631.
- "Bristol-Myers Squibb Announces Discontinuation of Development of Muraglitazar, an Investigational Oral Treatment for Type 2 Diabetes". PR Newswire from Bristol-Myers Squibb. May 18, 2006. Retrieved 9 November 2016.
- Nissen SE, Wolski K, Topol EJ (November 2005). "Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus". JAMA. 294 (20): 2581–6. doi:10.1001/jama.294.20.joc50147. PMID 16239637.
| PPARTooltip Peroxisome proliferator-activated receptor modulators | |
|---|---|
| PPARαTooltip Peroxisome proliferator-activated receptor alpha |
|
| PPARδTooltip Peroxisome proliferator-activated receptor delta | |
| PPARγTooltip Peroxisome proliferator-activated receptor gamma |
|
| Non-selective | |
| |
This drug article relating to the gastrointestinal system is a stub. You can help Misplaced Pages by expanding it. |