| |
| Names | |
|---|---|
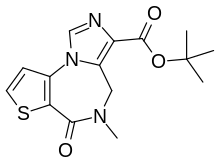
| IUPAC name tert-Butyl 8-methyl-7-oxo-5-thia-1,8,12-triazatricyclotrideca-2(6),3,10,12-tetraene-11-carboxylate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| IUPHAR/BPS | |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H17N3O3S |
| Molar mass | 319.38 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Ro 19-4603 is an inverse agonist of the benzodiazepine binding site. It has effects antagonistic to those of benzodiazepines.
Chemistry
Despite acting at the benzodiazepine site, it does not possess the benzodiazepine structure. It is an imidazothienodiazepine: a thiophene ring, an imidazole ring, and a diazepine ring fused together.
Effects and pharmacodynamics
Ro 19-4603 is an inverse agonist at the benzodiazepine binding site. Due to this, it has effects similar to other benzodiazepine inverse agonists, notably: anxiogenesis and convulsions.
In animal studies, administration of this compound was able to decrease voluntary alcohol consumption. This was observed in rats selected for high alcohol preference. In addition to decreasing its consumption, Ro 19-4603 is able to antagonize the intoxicating effects of alcohol.
References
- "Tert-butyl 5-methyl-6-oxo-5,6-dihydro-4h-imidazo[1,5-a]thieno[2,3-f][1,4]diazepine-3-carboxylate".
- Belzung, C.; Misslin, R.; Vogel, E. (July 1990). "Anxiogenic effects of a benzodiazepine receptor partial inverse agonist, RO 19-4603, in a light/dark choice situation". Pharmacology, Biochemistry, and Behavior. 36 (3): 593–596. doi:10.1016/0091-3057(90)90260-o. ISSN 0091-3057. PMID 2165618. S2CID 9881393.
- Kubová, H.; Mares, P. (October 1994). "Convulsant action of a benzodiazepine receptor agonist/inverse agonist Ro 19-4603 in developing rats". Naunyn-Schmiedeberg's Archives of Pharmacology. 350 (4): 393–397. doi:10.1007/BF00178957. ISSN 0028-1298. PMID 7845475. S2CID 1751486.
- Balakleevsky, A.; Colombo, G.; Fadda, F.; Gessa, G. L. (1990). "Ro 19-4603, a benzodiazepine receptor inverse agonist, attenuates voluntary ethanol consumption in rats selectively bred for high ethanol preference". Alcohol and Alcoholism (Oxford, Oxfordshire). 25 (5): 449–452. ISSN 0735-0414. PMID 1965120.
- Lister, R. G.; Durcan, M. J. (1989-03-13). "Antagonism of the intoxicating effects of ethanol by the potent benzodiazepine receptor ligand Ro 19-4603". Brain Research. 482 (1): 141–144. doi:10.1016/0006-8993(89)90551-9. ISSN 0006-8993. PMID 2539880. S2CID 22770686.