| |
| Names | |
|---|---|
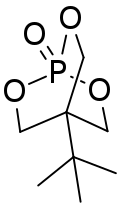
| Preferred IUPAC name 4-tert-Butyl-2,6,7-trioxa-1λ-phosphabicyclooctan-1-one | |
| Other names t-Butyl-bicyclophosphate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H15O4P |
| Molar mass | 206.178 g·mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Extremely toxic |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 36 μg/kg (mice) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
TBPO is an extremely toxic bicyclic phosphate convulsant and GABA receptor antagonist. It is the most toxic bicyclic phosphate known, with an LD50 of 36 μg/kg in mice.
Some sources claim that TBPO is as toxic as VX.
Synthesis
The synthesis is equivalent to the synthesis of IPTBO while the triol is produced by the condensation between 3,3-dimethylbutyraldehyde and formaldehyde analogous to the synthesis of trimethylolpropane.
See also
References
- Zhao, C.; Hwang, S. H.; Buchholz, B. A.; Carpenter, T. S.; Lightstone, F. C.; Yang, J.; Hammock, B. D.; Casida, J. E. (27 May 2014). "GABAA receptor target of tetramethylenedisulfotetramine". Proceedings of the National Academy of Sciences. 111 (23): 8607–8612. Bibcode:2014PNAS..111.8607Z. doi:10.1073/pnas.1407379111. PMC 4060666. PMID 24912155.
- Milbrath, Dean S.; Engel, Judith L.; Verkade, John G.; Casida, John E. (February 1979). "Structure-toxicity relationships of 1-substituted-4-alkyl-2,6,7-trioxabicyclooctanes". Toxicology and Applied Pharmacology. 47 (2): 287–293. Bibcode:1979ToxAP..47..287M. doi:10.1016/0041-008x(79)90323-5. PMID 452023.
- Gupta RC (2015). Handbook of toxicology of chemical warfare agents (2nd ed.). Amsterdam: Elsevier/Academic Press. pp. 228–229. ISBN 9780128004944. OCLC 433545336.
| Convulsants | |
|---|---|
| GABA receptor antagonists |
|
| GABA synthesis inhibitors | |
| Glycine receptor antagonists | |
| Glutamate receptor agonists | |
| Convulsant barbiturates | |
| Other | |
| Neurotoxins | |
|---|---|
| Animal toxins | |
| Bacterial | |
| Cyanotoxins | |
| Plant toxins | |
| Mycotoxins | |
| Pesticides | |
| Nerve agents | |
| Bicyclic phosphates | |
| Cholinergic neurotoxins | |
| Psychoactive drugs | |
| Other | |
This neurotoxin article is a stub. You can help Misplaced Pages by expanding it. |