| |
| Names | |
|---|---|
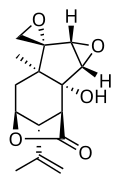
| IUPAC name (1S,2R,3S,5R,6R,7R,9S,12R)-2-Hydroxy-7-methyl-12-prop-1-en-2-ylspirododecane-6,2'-oxirane]-11-one | |
| Other names Coriamyrtine | |
| Identifiers | |
| 3D model (JSmol) | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H18O5 |
| Molar mass | 278.304 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Coriamyrtin is a toxic γ-lactone naturally present in a multitude of plants.
Natural occurrence
Coriamyrtin can be found in Scurrula parasitica, Coriaria microphylla, and certain other plants.
Toxicity
Coriamyrtin is a convulsant. It appears to act via antagonism of GABAA receptors. Poisoning is usually from ingestion of parts of the plants containing it. A case of poisoning was able to be treated with repeated administration of diazepam, an anticonvulsant.
References
- "Coriamyrtin".
- PubChem. "Scurrula parasitica". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-02-11.
- PubChem. "Coriaria microphylla". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-02-11.
- "T3DB: Coriamyrtin". www.t3db.ca. Retrieved 2024-02-11.
- Pérez, Claudia; Becerra, José; Manríquez-Navarro, Paula; Aguayo, Luis Gerardo; Fuentealba, Jorge; Guzmán, José Leonardo; Joseph-Nathan, Pedro; Jiménez, Verónica; Muñoz, Marcelo Andrés; Silva, Mario (2011). "Inhibitory activities on mammalian central nervous system receptors and computational studies of three sesquiterpene lactones from Coriaria ruscifolia subsp. ruscifolia". Chemical & Pharmaceutical Bulletin. 59 (2): 161–165. doi:10.1248/cpb.59.161. ISSN 1347-5223. PMID 21297293.
- de Haro, Luc; Pommier, Philip; Tichadou, Lucia; Hayek-Lanthois, Maryvonne; Arditti, Jocelyne (November 2005). "Poisoning by Coriaria myrtifolia Linnaeus: a new case report and review of the literature". Toxicon. 46 (6): 600–603. Bibcode:2005Txcn...46..600D. doi:10.1016/j.toxicon.2005.06.026. ISSN 0041-0101. PMID 16165183.
| Convulsants | |
|---|---|
| GABA receptor antagonists |
|
| GABA synthesis inhibitors | |
| Glycine receptor antagonists | |
| Glutamate receptor agonists | |
| Convulsant barbiturates | |
| Other | |
| Neurotoxins | |
|---|---|
| Animal toxins | |
| Bacterial | |
| Cyanotoxins | |
| Plant toxins | |
| Mycotoxins | |
| Pesticides | |
| Nerve agents | |
| Bicyclic phosphates | |
| Cholinergic neurotoxins | |
| Psychoactive drugs | |
| Other | |
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |