| Revision as of 02:48, 14 June 2011 editJohn (talk | contribs)Extended confirmed users215,415 edits →Synthesis and reactions: ce← Previous edit | Latest revision as of 00:27, 25 September 2023 edit undoOzzie10aaaa (talk | contribs)Autopatrolled, Extended confirmed users, New page reviewers213,559 editsm Cleaned up using AutoEd | ||
| (52 intermediate revisions by 35 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 434168427 | ||
| ⚫ | | |
||
| | ImageFile = Potassium-sulfide-unit-cell-3D-ionic.png | | Name = Potassium sulfide | ||
| | ImageFile = Potassium-sulfide-unit-cell-3D-ionic.png | |||
| ⚫ | | ImageName = Potassium sulfide | ||
| <!-- | ImageSize = 200px --> | |||
| | |
| ImageFile1 = Potassium sulfide.JPG | ||
| | |
| IUPACName = Potassium sulfide | ||
| | |
| OtherNames = Dipotassium monosulfide,<br />Dipotassium sulfide,<br />Potassium monosulfide,<br />Potassium sulfide | ||
| | |
|Section1={{Chembox Identifiers | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | CASNo = 1312-73-8 | |||
| | |
| CASNo = 1312-73-8 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 31R0R7HD0N | |||
| | RTECS = TT6000000 | |||
| | EC_number = 215-197-0 | |||
| | UNNumber = 1847 1382 | |||
| | PubChem = 162263 | |||
| | ChemSpiderID = 142491 | |||
| | SMILES = .. | |||
| | StdInChI = 1S/2K.S/q2*+1;-2 | |||
| | StdInChIKey = DPLVEEXVKBWGHE-UHFFFAOYSA-N | |||
| ⚫ | }} | ||
| |Section2={{Chembox Properties | |||
| ⚫ | | Formula = K<sub>2</sub>S | ||
| ⚫ | | MolarMass = 110.262 g/mol | ||
| ⚫ | | Appearance = pure: colourless<br />impure: yellow-brown | ||
| | Odor = ] | |||
| ⚫ | | Density = 1.74 g/cm<sup>3</sup> | ||
| ⚫ | | Solubility = converts to KSH, KOH | ||
| ⚫ | | Solvent = other solvents | ||
| ⚫ | | SolubleOther = soluble in ], ] <br> insoluble in ] | ||
| | MeltingPtC = 840 | |||
| | BoilingPtC = 912 | |||
| ⚫ | | BoilingPt_notes = (decomposes) | ||
| | MagSus = −60.0·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| }} | |||
| ⚫ | |Section3={{Chembox Structure | ||
| ⚫ | | CrystalStruct = anti] | ||
| ⚫ | }} | ||
| |Section4={{Chembox Thermochemistry | |||
| |DeltaHf=-406.2 kJ·mol<sup>−1</sup><ref name="jct">{{cite journal |last1=Johnson |first1=G.K. |last2=Steele |first2=W.V. |title=The standard enthalpy of formation of potassium sulfide (K<sub>2</sub>S) by fluorine bomb calorimetry |journal=The Journal of Chemical Thermodynamics |date=1981 |volume=13 |issue=10 |pages=985-990 |doi=10.1016/0021-9614(81)90075-6 |language=English}}</ref> | |||
| |DeltaGf=-392.4 kJ·mol<ref name="jct" /> | |||
| |Entropy=105.00 J·mol<sup>−1</sup>·K<sup>−1</sup><ref>{{cite book |title=CRC Handbook of Chemistry and Physics |date=2014 |publisher=CRC Press |isbn=1482208679 |pages=5-15 |edition=95th}}</ref> | |||
| }} | }} | ||
| | |
|Section7={{Chembox Hazards | ||
| | ExternalSDS = | |||
| ⚫ | | |
||
| ⚫ | | MainHazards = Causes skin burns. Dangerous for the environment | ||
| ⚫ | | |
||
| | GHSPictograms = {{GHS05}}{{GHS09}} | |||
| ⚫ | | |
||
| | GHSSignalWord = Danger | |||
| ⚫ | | |
||
| | HPhrases = {{H-phrases|314|400}} | |||
| ⚫ | | |
||
| | PPhrases = {{P-phrases|260|264|273|280|301+330+331|303+361+353|304+340|305+351+338|310|321|363|391|405|501}} | |||
| ⚫ | | |
||
| ⚫ | | |
||
| | MeltingPt = 840 °C | |||
| ⚫ | | |
||
| ⚫ | }} |
||
| |] | |||
| | ? °C | |||
| |- | |||
| --> | |||
| ⚫ | | |
||
| ⚫ | | |
||
| }} | }} | ||
| | |
|Section8={{Chembox Related | ||
| | OtherAnions = ]<br>]<br>]<br>] | |||
| | ExternalMSDS = | |||
| ⚫ | | OtherCations = ]<br>]<br>]<br>] | ||
| ⚫ | | |
||
| | OtherCompounds = ]<br>]<br>]<br>] | |||
| | RPhrases = {{R17}}, {{R23}}, {{R25}}, {{R31}}, {{R34}}, {{R50}} | |||
| | SPhrases = {{S24}}, {{S26}} | |||
| ⚫ | }} | ||
| | Section8 = {{Chembox Related | |||
| ⚫ | | |
||
| | OtherCpds = ], ] | |||
| }} | }} | ||
| }} | }} | ||
| ] | |||
| '''Potassium sulfide''' is |
'''Potassium sulfide''' is an ] with the formula ]<sub>2</sub>]. The colourless solid is rarely encountered, because it reacts readily with water, a reaction that affords ] (KSH) and potassium hydroxide (KOH). Most commonly, the term potassium sulfide refers loosely to this mixture, not the anhydrous solid. | ||
| ==Structure== | ==Structure== | ||
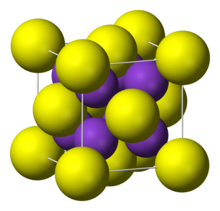
| It adopts "antifluorite structure," which means that the small K<sup>+</sup> ions occupy the tetrahedral (F<sup>−</sup>) sites in ], and the larger S<sup>2−</sup> centers occupy the eight-coordinate sites. ], ], and Rb<sub>2</sub>S crystallize similarly.<ref name=Holleman>Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN |
It adopts "antifluorite structure," which means that the small K<sup>+</sup> ions occupy the tetrahedral (F<sup>−</sup>) sites in ], and the larger S<sup>2−</sup> centers occupy the eight-coordinate sites. ], ], and Rb<sub>2</sub>S crystallize similarly.<ref name=Holleman>Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. {{ISBN|0-12-352651-5}}.</ref> | ||
| ==Synthesis and reactions== | ==Synthesis and reactions== | ||
| It can be produced by heating K<sub>2</sub>SO<sub>4</sub> with carbon (]): | It can be produced by ] K<sub>2</sub>SO<sub>4</sub> with carbon (]): | ||
| :K<sub>2</sub>SO<sub>4</sub> + 4 C → K<sub>2</sub>S + 4 CO | :K<sub>2</sub>SO<sub>4</sub> + 4 C → K<sub>2</sub>S + 4 CO | ||
| In the laboratory, |
In the laboratory, pure K<sub>2</sub>S may be prepared by the reaction of potassium and sulfur in anhydrous ammonia. <ref>Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 360.</ref> | ||
| Another method of making K<sub>2</sub>S in laboratory involves the reaction of ] and elemental sulfur: | |||
| : 2 KMnO<sub>4</sub> + S → K<sub>2</sub>S + 2 MnO<sub>2</sub> + 2 O<sub>2</sub> | |||
| Sulfide is highly basic, consequently K<sub>2</sub>S completely and irreversibly ] in water according to the following equation: | Sulfide is highly basic, consequently K<sub>2</sub>S completely and irreversibly ] in water according to the following equation: | ||
| Line 60: | Line 77: | ||
| ==Use in fireworks== | ==Use in fireworks== | ||
| Potassium sulfides are formed when ] is burned and are important intermediates in many pyrotechnic effects, such as senko hanabi and some glitter formulations.<ref name=Shimizu>Shimizu, Takeo. "Fireworks: the Art, Science, and Technique." Pyrotechnica Publications: Austin, 1981. ISBN |
Potassium sulfides are formed when ] is burned and are important intermediates in many pyrotechnic effects, such as ] and some ] formulations.<ref name=Shimizu>Shimizu, Takeo. "Fireworks: the Art, Science, and Technique." Pyrotechnica Publications: Austin, 1981. {{ISBN|0-929388-05-4}}.</ref> | ||
| ==See also== | ==See also== | ||
| *] | * ] | ||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| {{Potassium compounds}} | {{Potassium compounds}} | ||
| {{Sulfides}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 00:27, 25 September 2023
| |

| |
| Names | |
|---|---|
| IUPAC name Potassium sulfide | |
| Other names
Dipotassium monosulfide, Dipotassium sulfide, Potassium monosulfide, Potassium sulfide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.013.816 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1847 1382 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | K2S |
| Molar mass | 110.262 g/mol |
| Appearance | pure: colourless impure: yellow-brown |
| Odor | H2S |
| Density | 1.74 g/cm |
| Melting point | 840 °C (1,540 °F; 1,110 K) |
| Boiling point | 912 °C (1,674 °F; 1,185 K) (decomposes) |
| Solubility in water | converts to KSH, KOH |
| Solubility in other solvents | soluble in ethanol, glycerol insoluble in ether |
| Magnetic susceptibility (χ) | −60.0·10 cm/mol |
| Structure | |
| Crystal structure | antiFluorite |
| Thermochemistry | |
| Std molar entropy (S298) |
105.00 J·mol·K |
| Std enthalpy of formation (ΔfH298) |
-406.2 kJ·mol |
| Gibbs free energy (ΔfG) | -392.4 kJ·mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Causes skin burns. Dangerous for the environment |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H314, H400 |
| Precautionary statements | P260, P264, P273, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P391, P405, P501 |
| Related compounds | |
| Other anions | Potassium oxide Potassium selenide Potassium telluride Potassium polonide |
| Other cations | Lithium sulfide Sodium sulfide Rubidium sulfide Caesium sulfide |
| Related compounds | Potassium hydrosulfide Potassium sulfite Potassium sulfate Iron(II) sulfide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |

Potassium sulfide is an inorganic compound with the formula K2S. The colourless solid is rarely encountered, because it reacts readily with water, a reaction that affords potassium hydrosulfide (KSH) and potassium hydroxide (KOH). Most commonly, the term potassium sulfide refers loosely to this mixture, not the anhydrous solid.
Structure
It adopts "antifluorite structure," which means that the small K ions occupy the tetrahedral (F) sites in fluorite, and the larger S centers occupy the eight-coordinate sites. Li2S, Na2S, and Rb2S crystallize similarly.
Synthesis and reactions
It can be produced by heating K2SO4 with carbon (coke):
- K2SO4 + 4 C → K2S + 4 CO
In the laboratory, pure K2S may be prepared by the reaction of potassium and sulfur in anhydrous ammonia.
Sulfide is highly basic, consequently K2S completely and irreversibly hydrolyzes in water according to the following equation:
- K2S + H2O → KOH + KSH
For many purposes, this reaction is inconsequential since the mixture of SH and OH behaves as a source of S. Other alkali metal sulfides behave similarly.
Use in fireworks
Potassium sulfides are formed when black powder is burned and are important intermediates in many pyrotechnic effects, such as senko hanabi and some glitter formulations.
See also
References
- CRC Handbook of Chemistry and Physics (95th ed.). CRC Press. 2014. pp. 5–15. ISBN 1482208679.
- ^ Johnson, G.K.; Steele, W.V. (1981). "The standard enthalpy of formation of potassium sulfide (K2S) by fluorine bomb calorimetry". The Journal of Chemical Thermodynamics. 13 (10): 985–990. doi:10.1016/0021-9614(81)90075-6.
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 360.
- Shimizu, Takeo. "Fireworks: the Art, Science, and Technique." Pyrotechnica Publications: Austin, 1981. ISBN 0-929388-05-4.
| Potassium compounds | |
|---|---|
| H, (pseudo)halogens | |
| chalcogens | |
| pnictogens | |
| B, C group | |
| transition metals | |
| organic | |
| Sulfides (S) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||