| Revision as of 19:37, 11 February 2012 editAnypodetos (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers39,350 edits ATC code← Previous edit | Latest revision as of 07:15, 8 January 2025 edit undo38.49.72.163 (talk) →Indelible ink: "For over 1,000 years, beginning in the 14th century" is absurd: over 1000 years after the 14th century would be after the year 2300, which is almost 300 years in the future. Those two incompatible time markers were taken out of context from different, unrelated parts of the cited reference. I removed the absurd "over 1000 years" part and left the supportable "14th century" partTags: Mobile edit Mobile web edit | ||
| (351 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | |
|Watchedfields = changed | ||
| | |
|verifiedrevid = 476997183 | ||
| | |
|Name = Silver nitrate | ||
| | |
|ImageFile1 = Silver-nitrate-2D.svg | ||
| |ImageClass1 = skin-invert | |||
| | ImageName1 = Silver nitrate | |||
| |ImageSize1 = 160px | |||
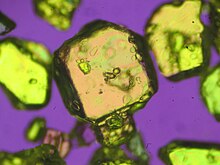
| | ImageFile2 = Silver nitrate crystals.jpg | |||
| | |
|ImageName1 = Structural formula of silver nitrate | ||
| |ImageCaption1 = ] | |||
| | ImageSize2 = 120px | |||
| |ImageFile2 = Silver nitrate crystals.jpg | |||
| | OtherNames = Nitric acid silver(1+) salt | |||
| |ImageSize2 = 160px | |||
| | Section1 = {{Chembox Identifiers | |||
| |ImageName2 = Sample of silver nitrate | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| |ImageFile3 = Silver-nitrate-xtal-2x2x2-c-3D-bs-17.png | |||
| | ChemSpiderID = 22878 | |||
| |ImageName3 = Crystal structure of silver nitrate | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| |ImageCaption3 = ] | |||
| | ChEMBL = 177367 | |||
| |IUPACName = Silver nitrate | |||
| | PubChem = 24470 | |||
| |OtherNames = Nitric acid silver(1+) salt <br /> Lapis infernalis <br /> Argentous nitrate | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| |SystematicName = Silver(I) nitrate | |||
| | UNII = 95IT3W8JZE | |||
| |Section1 = {{Chembox Identifiers | |||
| | InChI = 1/Ag.NO3/c;2-1(3)4/q+1;-1 | |||
| |ChemSpiderID = 22878 | |||
| | InChIKey = SQGYOTSLMSWVJD-UHFFFAOYAW | |||
| |ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | SMILES = (=O)(). | |||
| |ChEMBL = 177367 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| |ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | StdInChI = 1S/Ag.NO3/c;2-1(3)4/q+1;-1 | |||
| |PubChem = 24470 | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| |UNII = 95IT3W8JZE | |||
| | StdInChIKey = SQGYOTSLMSWVJD-UHFFFAOYSA-N | |||
| |UNII_Ref = {{fdacite|correct|FDA}} | |||
| | CASNo = 7761-88-8 | |||
| |InChI = 1/Ag.NO3/c;2-1(3)4/q+1;-1 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| |InChIKey = SQGYOTSLMSWVJD-UHFFFAOYAW | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| |SMILES = (=O)(). | |||
| | ChEBI = 32130 | |||
| |StdInChI = 1S/Ag.NO3/c;2-1(3)4/q+1;-1 | |||
| | ATCCode_prefix = D08 | |||
| |StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | ATCCode_suffix = AL01 | |||
| |StdInChIKey = SQGYOTSLMSWVJD-UHFFFAOYSA-N | |||
| }} | |||
| |StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | Section2 = {{Chembox Properties | |||
| |CASNo = 7761-88-8 | |||
| | Ag = 1|N = 1|O = 3 | |||
| |CASNo_Ref = {{cascite|correct|CAS}} | |||
| | Appearance = white solid | |||
| |ChEBI = 32130 | |||
| | Density = 4.35 g/cm<sup>3</sup> | |||
| |ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | MeltingPtC = 212 | |||
| |RTECS = VW4725000 | |||
| | BoilingPtC = 444 | |||
| |UNNumber = 1493 | |||
| | Boiling_notes = ''decomp.'' | |||
| |EC_number = 231-853-9 | |||
| | Solubility = 1.22 kg/L (0 °C) <br /> 2.16 kg/L (20 °C) <br /> 4.40 kg/L (60 °C) <br /> 7.33 kg/L (100 °C) | |||
| | SolubleOther = soluble in ] and ] | |||
| }} | |||
| | Section7 = {{Chembox Hazards | |||
| | EUClass = {{Hazchem C}}<br />{{Hazchem N}} | |||
| | NFPA-H = 2 | |||
| | NFPA-F = 0 | |||
| | NFPA-R = 0 | |||
| | NFPA-O = | |||
| | RPhrases = {{R8}},{{R34}}, {{R50/53}} | |||
| | SPhrases = {{S1/2}}, {{S26}}, {{S45}}, {{S60}}, {{S61}} | |||
| }} | |||
| }} | }} | ||
| |Section2 = {{Chembox Properties | |||
| '''Silver nitrate''' is an ] with ] {{chem|AgNO|3}}. This compound is a versatile precursor to many other ] compounds, such as those used in ]. It is far less sensitive to light than the ]. It was once called ''lunar caustic'' because silver was called ''luna'' by the ancient alchemists, because they believed that silver was associated with the moon.<ref>{{cite web|url=http://dictionary.die.net/lunar%20caustic |title=Definition of Lunar Caustic}}</ref> | |||
| |Ag=1 | N=1 | O=3 | |||
| |Appearance = colorless solid | |||
| |Odor = Odorless | |||
| |Density = 4.35 g/cm<sup>3</sup> (24 °C)<br> 3.97 g/cm<sup>3</sup> (210 °C)<ref name=crc>{{CRC90}}</ref> | |||
| |MeltingPtC = 209.7 | |||
| |MeltingPt_ref = <ref name=crc /><ref name=chemister /> | |||
| |BoilingPtC = 440 | |||
| |BoilingPt_notes = <br> decomposes<ref name=crc /> | |||
| |Solubility = 122 g/100 mL (0 °C)<br> 170 g/100 mL (10 °C)<br> 256 g/100 mL (25 °C)<br> 373 g/100 mL (40 °C)<br> 912 g/100 mL (100 °C)<ref name=sioc>{{cite book|last1 = Seidell|first1 = Atherton|last2 = Linke|first2 = William F.|year = 1919|title = Solubilities of Inorganic and Organic Compounds|url = https://archive.org/details/solubilitiesino04seidgoog|publisher = D. Van Nostrand Company|place = ]|edition = 2nd|pages = –619}}</ref> | |||
| |SolubleOther = Soluble in ],<ref name=crc /> ], ], ] | |||
| |Solubility1 = 0.776 g/kg (30 °C)<br> 1.244 g/kg (40 °C)<br> 5.503 g/kg (93 °C)<ref name=chemister>{{cite web|last = Kiper |first = Ruslan Anatolievich|website = Chemister.ru |url = http://chemister.ru/Database/properties-en.php?dbid=1&id=862|title = silver nitrate|access-date = 2014-07-20}}</ref> | |||
| |Solvent1 = acetic acid | |||
| |Solubility2 = 0.35 g/100 g (14 °C)<br> 0.44 g/100 g (18 °C)<ref name=sioc /> | |||
| |Solvent2 = acetone | |||
| |Solubility3 = 0.22 g/kg (35 °C)<br> 0.44 g/kg (40.5 °C)<ref name=sioc /> | |||
| |Solvent3 = benzene | |||
| |Solubility4 = 3.1 g/100 g (19 °C)<ref name=sioc /> | |||
| |Solvent4 = ethanol | |||
| |Solubility5 = 2.7 g/100 g (20 °C)<ref name=chemister /> | |||
| |Solvent5 = ethyl acetate | |||
| |RefractIndex = 1.744 | |||
| |Viscosity = 3.77 ] (244 °C)<br> 3.04 cP (275 °C)<ref name=chemister /> | |||
| |LogP = 0.19 | |||
| |MagSus = −45.7·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| }} | |||
| |Section3 = {{Chembox Structure | |||
| |CrystalStruct = Orthorhombic, ]<ref name=iucr /> | |||
| |SpaceGroup = P2<sub>1</sub>2<sub>1</sub>2<sub>1</sub>, No. 19<ref name=iucr /> | |||
| |PointGroup = 222<ref name=iucr /> | |||
| |LattConst_a = 6.992(2) Å | |||
| |LattConst_b = 7.335(2) Å | |||
| |LattConst_c = 10.125(2) Å<ref name=iucr /> | |||
| |LattConst_alpha = 90 | |||
| }} | |||
| |Section4 = {{Chembox Thermochemistry | |||
| |DeltaHf = −124.4 kJ/mol<ref name=crc /> | |||
| |DeltaGf = −33.4 kJ/mol<ref name=crc /> | |||
| |Entropy = 140.9 J/mol·K<ref name=crc /> | |||
| |HeatCapacity = 93.1 J/mol·K<ref name=crc /> | |||
| }} | |||
| |Section5 = {{Chembox Pharmacology | |||
| |ATCCode_prefix = D08 | |||
| |ATCCode_suffix = AL01 | |||
| }} | |||
| |Section6 = {{Chembox Hazards | |||
| |GHSPictograms = {{GHS03}}{{GHS05}}{{GHS06}}{{GHS09}}<ref name="sigma">{{Sigma-Aldrich|id = 204390|name = Silver nitrate|accessdate = 2014-07-20}}</ref> | |||
| |GHSSignalWord = Danger | |||
| |HPhrases = {{H-phrases|272|314|410}}<ref name="sigma" /> | |||
| |PPhrases = {{P-phrases|220|273|280|305+351+338|310|501}}<ref name="sigma" /> | |||
| |NFPA-H = 3 | |||
| |NFPA-F = 0 | |||
| |NFPA-R = 2 | |||
| |NFPA-S = OX | |||
| |MainHazards = Reacts explosively with ethanol. Toxic. Corrosive. | |||
| |LDLo = 800 mg/kg (rabbit, oral)<br />20 mg/kg (dog, oral)<ref>{{IDLH|7440224|Silver (metal dust and soluble compounds, as Ag)}}</ref> | |||
| }} | |||
| }} | |||
| ] | |||
| In solid silver ], the silver ions are three-coordinated in a trigonal planar arrangement.<ref>{{cite journal |
'''Silver nitrate''' is an ] with ] {{chem|AgNO|3}}. It is a versatile ] to many other ] compounds, such as those used in ]. It is far less sensitive to light than the ].{{Citation needed|date=July 2024}} It was once called ''lunar caustic'' because silver was called ''luna'' by ancient alchemists who associated silver with the ].<ref>{{cite web|url=http://dictionary.die.net/lunar%20caustic|title=Definition of Lunar Caustic|work=dictionary.die.net|url-status=dead|archive-url=https://web.archive.org/web/20120131215637/http://dictionary.die.net/lunar%20caustic|archive-date=2012-01-31}}</ref> In solid silver ], the silver ions are three-] in a trigonal planar arrangement.<ref name=iucr>{{cite journal | title = Structure du nitrate d'argent à pression et température ordinaires. Exemple de cristal parfait | first1 = P. | last1 = Meyer | first2 = A. | last2 = Rimsky | first3 = R. | last3 = Chevalier | journal = ] | year = 1978 | volume = 34 | issue = 5 | pages = 1457–1462 | doi = 10.1107/S0567740878005907 | bibcode = 1978AcCrB..34.1457M }}</ref> | ||
| ==Synthesis and structure== | |||
| ==Discovery== | |||
| ], in the 13th century, documented the ability of ] to separate ] and ] by dissolving the silver.<ref>{{cite book|last = Szabadváry|first = Ferenc|title = History of analytical chemistry|publisher = Taylor & Francis|year = 1992| |
], in the 13th century, documented the ability of ] to separate ] and ] by dissolving the silver.<ref>{{cite book|last = Szabadváry|first = Ferenc|title = History of analytical chemistry|publisher = Taylor & Francis|year = 1992|page = 17|url = https://books.google.com/books?id=53APqy0KDaQC&pg=PA17|isbn = 978-2-88124-569-5}}</ref> Indeed silver nitrate can be prepared by dissolving silver in ] followed by evaporation of the solution. The stoichiometry of the reaction depends upon the concentration of nitric acid used. | ||
| :3 Ag + 4 HNO<sub>3</sub> (cold and diluted) → 3 AgNO<sub>3</sub> + 2 H<sub>2</sub>O + ] | |||
| :Ag + 2 HNO<sub>3</sub> (hot and concentrated) → AgNO<sub>3</sub> + H<sub>2</sub>O + ] | |||
| The structure of silver nitrate has been examined by ] several times. In the common orthorhombic form stable at ordinary temperature and pressure, the silver atoms form pairs with Ag---Ag contacts of 3.227 Å. Each Ag<sup>+</sup> center is bonded to six oxygen centers of both uni- and bidentate nitrate ligands. The Ag-O distances range from 2.384 to 2.702 Å.<ref name=iucr /> | |||
| ==Synthesis== | |||
| Silver nitrate can be prepared by reacting silver, such as a silver bullion or silver foil, with ]: | |||
| :3 Ag + 4 {{chem|HNO|3}} → 3 {{chem|AgNO|3}} + 2 {{chem|H|2|O}} + {{chem|NO|}} | |||
| ] | |||
| This is performed under a fume hood because of toxic nitrogen oxide(s) evolved during the reaction.<ref>{{cite web|title = Making silver nitrate (youtube)|url = http://www.youtube.com/watch?v=d6hPgGV_qAg&feature=channel_page}}</ref> | |||
| ==Reactions== | ==Reactions== | ||
| A typical reaction with silver nitrate is to suspend a rod of ] in a solution of silver nitrate and leave it for a few hours. The silver nitrate reacts with copper to form hairlike crystals of silver metal and a blue solution of copper nitrate: | A typical reaction with silver nitrate is to suspend a rod of ] in a solution of silver nitrate and leave it for a few hours. The silver nitrate reacts with copper to form hairlike crystals of silver metal and a blue solution of ]: | ||
| : 2 AgNO<sub>3</sub> + Cu → Cu(NO<sub>3</sub>)<sub>2</sub> + 2 Ag | : 2 AgNO<sub>3</sub> + Cu → Cu(NO<sub>3</sub>)<sub>2</sub> + 2 Ag | ||
| Silver nitrate |
Silver nitrate decomposes when heated: | ||
| : 2 AgNO<sub>3</sub>(l) → 2 ](s) + ](g) + 2 ](g) | |||
| Qualitatively, decomposition is negligible below the melting point, but becomes appreciable around 250 °C and fully decomposes at 440 °C.<ref>{{Cite journal | doi = 10.1063/1.3253104| title = High Temperature Properties and Decomposition of Inorganic Salts Part 3, Nitrates and Nitrites| journal = Journal of Physical and Chemical Reference Data| volume = 1| issue = 3| pages = 747–772| year = 1972| last1 = Stern | first1 = K. H. | bibcode = 1972JPCRD...1..747S| s2cid = 95532988}}</ref> | |||
| : 2 AgNO<sub>3</sub> → 2 Ag + O<sub>2</sub> + 2 NO<sub>2</sub> | |||
| Most metal nitrates thermally decompose to the respective |
Most metal nitrates thermally decompose to the respective ]s, but ] decomposes at a lower temperature than silver nitrate, so the decomposition of silver nitrate yields elemental silver instead. | ||
| ==Uses== | ==Uses== | ||
| ===Precursor to other silver compounds=== | ===Precursor to other silver compounds=== | ||
| Silver nitrate is the least expensive salt of silver; it offers several other advantages as well. It is non-], in contrast to ] and ]. |
Silver nitrate is the least expensive ] of silver; it offers several other advantages as well. It is non-], in contrast to ] and ]. In addition, it is relatively stable to light, and it dissolves in numerous solvents, including water. The nitrate can be easily replaced by other ], rendering AgNO<sub>3</sub> versatile. Treatment with solutions of halide ions gives a precipitate of AgX (X = Cl, Br, I). When making ], silver nitrate is treated with ] salts of sodium or potassium to form insoluble ] in situ in photographic ], which is then applied to strips of tri-] or ]. Similarly, silver nitrate is used to prepare some silver-based explosives, such as the ], ], or ], through a ]. | ||
| Treatment of silver nitrate with base gives dark grey ]:<ref>{{OrgSynth|author = Campaigne, E.; LeSuer, W. M.|title = 3-Thiophenecarboxylic (Thenoic) Acid|collvol = 4|collvolpages = 919|year = 1963|prep = cv4p0919}} (preparation of Ag<sub>2</sub>O, used in oxidation of an aldehyde)</ref> | Treatment of silver nitrate with base gives dark grey ]:<ref>{{OrgSynth|author = Campaigne, E.; LeSuer, W. M.|title = 3-Thiophenecarboxylic (Thenoic) Acid|collvol = 4|collvolpages = 919|year = 1963|prep = cv4p0919}} (preparation of Ag<sub>2</sub>O, used in oxidation of an aldehyde)</ref> | ||
| :2 AgNO<sub>3</sub> + 2 NaOH → Ag<sub>2</sub>O + 2 NaNO<sub>3</sub> + H<sub>2</sub>O | :2 AgNO<sub>3</sub> + 2 NaOH → Ag<sub>2</sub>O + 2 NaNO<sub>3</sub> + H<sub>2</sub>O | ||
| Standard solution molarity = 0.05006 M, depends on solubility and concentration however. | |||
| ===Halide abstraction=== | ===Halide abstraction=== | ||
| The silver cation, {{chem|Ag|+}}, reacts quickly with halide sources to produce the insoluble silver halide, which is a cream precipitate if Br- is used, a white precipitate if Cl- is used and a yellow precipitate if I- is used. This reaction is commonly used in ] to abstract halides: | The silver cation, {{chem|Ag|+}}, reacts quickly with halide sources to produce the insoluble silver halide, which is a cream precipitate if {{chem|Br|-}} is used, a white precipitate if {{chem|Cl|-}} is used and a yellow precipitate if {{chem|I|-}} is used. This reaction is commonly used in ] to abstract halides: | ||
| :{{chem|Ag|+}} + {{chem|X|-}} |
:{{chem|Ag|+}}(aq) + {{chem|X|-}}(aq) → AgX(s) | ||
| where {{chem|X|-}} = {{chem|Cl|-}}, {{chem|Br|-}}, or {{chem|I|-}}. | where {{chem|X|-}} = {{chem|Cl|-}}, {{chem|Br|-}}, or {{chem|I|-}}. | ||
| Line 93: | Line 139: | ||
| Other silver salts with ], namely ] and ] are used for more demanding applications. | Other silver salts with ], namely ] and ] are used for more demanding applications. | ||
| Similarly, this reaction is used in ] to confirm the presence of ], ], or ] ] |
Similarly, this reaction is used in ] to confirm the presence of ], ], or ] ]. Samples are typically acidified with dilute nitric acid to remove interfering ions, e.g. ] ions and ] ions. This step avoids confusion of ] or ] precipitates with that of silver halides. The color of precipitate varies with the halide: white (]), pale yellow/cream (]), yellow (]). AgBr and especially AgI ] to the metal, as evidenced by a grayish color on exposed samples. | ||
| The same reaction was used on steamships in order to determine whether or not ] had been contaminated with ]. It is still used to determine if moisture on formerly dry cargo is a result of ] from humid air, or from seawater leaking through the hull.<ref>{{cite web| title =Silver nitrate method| website =Transport Information Service| publisher =Gesamtverband der Deutschen Versicherungswirtschaf| url =http://www.tis-gdv.de/tis_e/misc/silber.htm| access-date = 22 June 2015 }}</ref> | |||
| ===Organic synthesis=== | ===Organic synthesis=== | ||
| Silver nitrate is used in many ways in ], e.g. for ] and oxidations. Ag |
Silver nitrate is used in many ways in ], e.g. for ] and oxidations. {{chem|Ag|+}} binds ]s reversibly, and silver nitrate has been used to separate mixtures of alkenes by selective absorption. The resulting ] can be decomposed with ] to release the free alkene.<ref>{{OrgSynth|author = Cope, A. C.; Bach, R. D.|title = trans-Cyclooctene|collvol = 5|collvolpages = 315|year = 1973|prep = cv5p0315}}</ref> Silver nitrate is highly soluble in water but is poorly soluble in most organic solvents, except ] (111.8 g/100 g, 25 °C).<ref>{{Cite web|url=http://chemister.ru/Database/properties-en.php?id=862|title=silver nitrate|website=chemister.ru|access-date=2019-04-04}}</ref> | ||
| ===Biology=== | ===Biology=== | ||
| In ], silver nitrate is used for ]ing, for demonstrating reticular fibers, ]s and ]s. For this reason it is also used to demonstrate proteins in ] gels. It |
In ], silver nitrate is used for ]ing, for demonstrating reticular fibers, ]s and ]s. For this reason it is also used to demonstrate proteins in ] gels. It can be used as a stain in ].<ref>{{cite journal|author = Geissinger HD|title = The use of silver nitrate as a stain for scanning electron microscopy of arterial intima and paraffin sections of kidney|journal = Journal of Microscopy|volume = 95|issue = 3|pages = 471–481|year = 2011|doi = 10.1111/j.1365-2818.1972.tb01051.x|pmid = 4114959|s2cid = 38335416}}</ref> | ||
| Cut flower stems can be placed in a silver nitrate solution, which prevents the production of ethylene. This delays ageing of the flower.<ref>{{Cite web |title=Silver Nitrate (072503) Fact Sheet |url=https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/fs_PC-072503_01-Oct-01.pdf |access-date=3 October 2024 |website=epa.gov}}</ref> | |||
| ===Antimicrobial uses=== | |||
| === Indelible ink === | |||
| *Water disinfection in hotels and hospitals{{Citation needed|date=April 2011}} | |||
| Silver nitrate produces long-lasting stain when applied to skin and is one of indelible ink’s ingredients. An ] makes use of this to mark a finger of people who have voted in an election, allowing easy identification to prevent double-voting.<ref>{{Cite news |date=2023-06-17 |title=The ink with a 'secret formula' that powers the world's biggest democratic exercise {{!}} India {{!}} The Guardian |newspaper=The Guardian |url=https://www.theguardian.com/world/2019/mar/28/india-the-ink-with-a-secret-formula-that-powers-the-worlds-biggest-democratic-exercise |access-date=2024-04-17 |archive-url=https://web.archive.org/web/20230617165311/https://www.theguardian.com/world/2019/mar/28/india-the-ink-with-a-secret-formula-that-powers-the-worlds-biggest-democratic-exercise |archive-date=2023-06-17 |last1=Dhillon |first1=Amrit }}</ref><ref>{{Cite news |last=Dhillon |first=Amrit |date=2019-03-28 |title=The ink with a 'secret formula' that powers the world's biggest democratic exercise |url=https://www.theguardian.com/world/2019/mar/28/india-the-ink-with-a-secret-formula-that-powers-the-worlds-biggest-democratic-exercise |access-date=2024-04-17 |work=The Guardian |language=en-GB |issn=0261-3077}}</ref> | |||
| *Postharvest cleaning of oysters and crabs{{Citation needed|date=April 2011}} | |||
| {{anchor|medical}} | |||
| *Inhibition of bacterial growth on chicken farms{{Citation needed|date=April 2011}} | |||
| *Water recycling aboard space shuttles{{Citation needed|date=April 2011}} | |||
| In addition to staining skin, silver nitrate has a history of use in stained glass. In the 14th century, artists began using a "silver stain" (also known as a yellow stain) made from silver nitrate to create a yellow effect on clear glass. The stain would produce a stable color that could range from pale lemon to deep orange or gold. Silver stain was often used with glass paint, and was applied to the opposite side of the glass as the paint. It was also used to create a mosaic effect by reducing the number of pieces of glass in a window. Despite the age of the technique, this process of creating stained glass remains almost entirely unchanged.<ref>{{Cite web |title=Khan Academy |url=https://www.khanacademy.org/humanities/medieval-world/gothic-art/beginners-guide-gothic-art/a/stained-glass-history-and-technique#:~:text=Why%20is%20it%20called%20stained,made%20the%20same%20way%20today. |access-date=2024-10-29 |website=www.khanacademy.org |language=en}}</ref> | |||
| *Home purification of water in Europe and North America{{Citation needed|date=April 2011}} | |||
| *Point of use disinfectant for water and vegetables in Mexico{{Citation needed|date=April 2011}} | |||
| *Alternative to antibiotics (not recommended by the FDA){{Citation needed|date=April 2011}} | |||
| *Alternative to laundry detergent{{Citation needed|date=April 2011}} | |||
| *Application to eyes of newborn babies to prevent infection{{Citation needed|date=April 2011}} | |||
| *Coating on catheters to prevent infection<ref name="Gupta">{{cite journal|last1 = Gupta|first1 = Amit | |||
| |last2 = Silver|first2 = Simon |year = 1998|title = Silver As a Biocide: Will Resistance Become a Problem?|journal = ] |volume = 16 |pages = 888|doi = 10.1038/nbt1098-888|pmid = 9788326|issue = 10}}</ref> | |||
| ==Medicine== | ==Medicine== | ||
| {{see also|Medical uses of silver}} | |||
| ] showing a silver nitrate (brown) marked ].]] | |||
| ] showing a silver nitrate (brown) marked ].]] | |||
| Silver salts have ] properties. Until the development and widespread adoption of antibiotics, dilute solutions of AgNO<sub>3</sub> used to be dropped into ]' eyes at birth to prevent contraction of ] from the mother. Eye infections and blindness of newborns was reduced by this method; incorrect dosage, however, could cause blindness in extreme cases. This protection was first used by ] in 1881.<ref>{{cite journal| author = Peter.H| title = Dr Carl Credé (1819-1892) and the prevention of ophthalmia neonatorum| journal = Arch Dis Child Fetal Neonatal| volume = 83| year = 2000| pages = F158–F159| doi = 10.1136/fn.83.2.F158| pmid = 10952715| issue = 2| pmc = 1721147}}</ref><ref>{{cite journal| author = Credé C. S. E.| title = Die Verhürtung der Augenentzündung der Neugeborenen| journal = Archiv für Gynaekologie| volume = 17| year = 1881| issue = 1| pages = 50–53| doi = 10.1007/BF01977793}}</ref><ref></ref> Fused silver nitrate, shaped into sticks, was traditionally called "lunar caustic". It is used as a ] agent, for example to remove ] around a ]. General Sir James Abbott noted in his journals that in India in 1827 it was infused by a British surgeon into wounds in his arm resulting from the bite of a mad dog to cauterize the wounds and prevent the onset of rabies. <ref> British Library, India Office Records, European Manuscripts, MSS EUR F171/33/3, page 109. </ref> Dentists sometimes use silver nitrate infused swabs to heal ]s. Silver nitrate is also used by some podiatrists to kill cells located in the nail bed. Silver nitrate is also used to cauterize superficial blood vessels in the nose to help prevent nose bleeds. | |||
| Silver salts have ] properties. In 1881 ] introduced a method known as ], which used of dilute (2%) solutions of silver nitrate in ]' eyes at birth to prevent contraction of ] from the mother, which could cause blindness via ]. (Modern antibiotics are now used instead).<ref name="Mate2013">{{cite journal|last1=Matejcek|first1=A|last2=Goldman|first2=RD|title=Treatment and prevention of ophthalmia neonatorum.|journal=Canadian Family Physician|date=November 2013|volume=59|issue=11|pages=1187–90|pmid=24235191|pmc=3828094}}</ref> <ref>{{cite journal| author = Peter.H| title = Dr Carl Credé (1819–1892) and the prevention of ophthalmia neonatorum| journal = Arch Dis Child Fetal Neonatal Ed| volume = 83| year = 2000| pages = F158–F159| doi = 10.1136/fn.83.2.F158| pmid = 10952715| issue = 2| pmc = 1721147}}</ref><ref>{{cite journal| author = Credé C. S. E.| title = Die Verhürtung der Augenentzündung der Neugeborenen| journal = Archiv für Gynäkologie| volume = 17| year = 1881| issue = 1| pages = 50–53| doi = 10.1007/BF01977793| s2cid = 10053605}}</ref><ref name=Ulrich>{{cite journal|url=https://www.scielosp.org/article/bwho/2001.v79n3/262-266/en/|journal=Bulletin of the World Health Organization|title=Is Credés prophylaxis for ophthalmia neonatorum still valid?|author1=Schaller, Ulrich C. |author2=Klauss, Volker |name-list-style=amp |volume=79 |issue=3|year=2001|pages=262–266|pmid=11285676|pmc=2566367}}</ref> | |||
| Fused silver nitrate, shaped into sticks, was traditionally called "lunar caustic". It is used as a ] agent, for example to remove ] around a ]. General ] noted in his journals that in India in 1827 it was infused by a British surgeon into wounds in his arm resulting from the bite of a mad dog to cauterize the wounds and prevent the onset of rabies.<ref>British Library, India Office Records, European Manuscripts, MSS EUR F171/33/3, page 109.</ref> | |||
| The Canadian physician C. A. Douglas Ringrose researched the use of silver nitrate for ] on women. A specialist in obstetrics and gynaecology, Ringrose believed that the corrosive properties of silver nitrate could be used to block and corrode the fallopian tubes, in a process that he called "office tubal sterilization".<ref>{{cite journal|journal=Obstetrics and Gynecology|volume=42|issue=1|pages=151–5|year=1973 |author=Ringrose CA.|title=Office tubal sterilization|pmid=4720201}}</ref> The technique was ineffective; in fact at least two women underwent abortions. Ringrose was sued for malpractice, although these suits were unsuccessful.<ref>Cryderman v. Ringrose (1978), 89 D.L.R. (3d) 32 (Alta S.C.) and Zimmer et al. v. Ringrose (1981) 4 W.W.R. 75 (Alta C.A.).</ref> | |||
| Silver nitrate is used to cauterize superficial blood vessels in the nose to help prevent ]s. | |||
| ===Disinfection=== | |||
| Much research has been done in evaluating the ability of the silver ion at inactivating ], a microorganism commonly used as an indicator for fecal contamination and as a surrogate for pathogens in drinking water treatment. Concentrations of silver nitrate evaluated in inactivation experiments range from 10–200 micrograms per liter as Ag<sup>+</sup>. The antimicrobial properties of silver was first observed thousands of years ago when silver containers were used to store water for preservation. Its disinfection ability has been scientifically studied for over a century. | |||
| Dentists sometimes use silver nitrate-infused swabs to heal ]s. Silver nitrate is used by some ]s to kill cells located in the nail bed. | |||
| Silver's antimicrobial activity saw many applications prior to the discovery of pharmaceutical antibiotics, when it fell into near disuse. Its association with ] made consumers wary and led them to turn away from it when given an alternative. Since that time, as antibiotic-resistant microorganisms have emerged, interest in using the silver ion for anti-microbial purposes has resumed.<ref name="colloidal">{{cite web | |||
| |title = A Brief History of the Health Support Uses of Silver | |||
| | publisher = Silver Colloids|url = http://www.silver-colloids.com/Pubs/history-silver.html | |||
| }}</ref> | |||
| The Canadian physician C. A. Douglas Ringrose researched the use of silver nitrate for ], believing that silver nitrate could be used to block and corrode the fallopian tubes.<ref>{{cite journal|journal=Obstetrics and Gynecology|volume=42|issue=1|pages=151–5|year=1973 |author=Ringrose CA.|title=Office tubal sterilization|pmid=4720201}}</ref> The technique was ineffective.<ref>Cryderman v. Ringrose (1978), 89 D.L.R. (3d) 32 (Alta S.C.) and Zimmer et al. v. Ringrose (1981) 4 W.W.R. 75 (Alta C.A.).</ref> | |||
| ====Kinetics==== | |||
| Before a disinfectant can be effectively used as a water disinfectant, its ] must be established. Kinetics generally depend on both the dosage of disinfectant and the time of application. It is important to understand the kinetics so that the minimum dosage of disinfectant can be applied for the minimum amount of time while still effectively inactivating any pathogens in the water. Because there are many microorganisms present in water, the inactivation kinetics of each one cannot be studied extensively. Therefore, indicator organisms generally more resistant to inactivation than others are used to estimate the kinetics of microorganisms as a whole. ''Escherichia coli,'' also referred to as ''E. coli'', is a commonly used indicator organism. | |||
| ===Disinfection=== | |||
| It is well documented that the silver ion is effective in the inactivation of ''E. coli''.<ref name="Potapchenko">Potapchenko, N. G., L. V. Grigor'eva, O. S. Savluk, and L. A. Kul'skii. "Dosage-Time Dependency of Effect of Silver in Water on Pathogenic Escherichia." Soviet Journal of Water Chemistry and Technology 10 (1988): 101-104.</ref><ref name="Hwang">{{cite journal|doi=10.1016/j.watres.2007.05.052|title=Inactivation of Legionella pneumophila and Pseudomonas aeruginosa: Evaluation of the bactericidal ability of silver cations|year=2007|last1=Hwang|first1=Myoung Goo|last2=Katayama|first2=Hiroyuki|last3=Ohgaki|first3=Shinichiro|journal=Water Research|volume=41|pages=4097|pmid=17606286|issue=18}}</ref><ref name = "Jung">{{cite journal|pmid=18245232|year=2008|last1=Jung|first1=WK|last2=Koo|first2=HC|last3=Kim|first3=KW|last4=Shin|first4=S|last5=Kim|first5=SH|last6=Park|first6=YH|title=Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli.|volume=74|issue=7|pages=2171–8|doi=10.1128/AEM.02001-07|pmc=2292600|journal=Applied and environmental microbiology}}</ref><ref name="Kim">{{cite journal|pmid=17692890|year=2008|last1=Kim|first1=JY|last2=Lee|first2=C|last3=Cho|first3=M|last4=Yoon|first4=J|title=Enhanced inactivation of E. coli and MS-2 phage by silver ions combined with UV-A and visible light irradiation.|volume=42|issue=1-2|pages=356–62|doi=10.1016/j.watres.2007.07.024|journal=Water research}}</ref><ref name= "Yamanaka">{{cite journal|doi=10.1128/AEM.71.11.7589-7593.2005|title=Bactericidal Actions of a Silver Ion Solution on Escherichia coli, Studied by Energy-Filtering Transmission Electron Microscopy and Proteomic Analysis|year=2005|last1=Yamanaka|first1=M.|last2=Hara|first2=K.|last3=Kudo|first3=J.|journal=Applied and Environmental Microbiology|volume=71|pages=7589|pmid=16269810|issue=11|pmc=1287701}}</ref><ref name = "Matsumura">{{cite journal|pmid=12839814|year=2003|last1=Matsumura|first1=Y|last2=Yoshikata|first2=K|last3=Kunisaki|first3=S|last4=Tsuchido|first4=T|title=Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate.|volume=69|issue=7|pages=4278–81|pmc=165194|journal=Applied and environmental microbiology|doi=10.1128/AEM.69.7.4278-4281.2003}}</ref><ref name="Khaydarov">Khaydarov, R. A., R. R. Khaydarov, R. L. Olsen, and S. E. Rogers. Journal of Water Supply: Research and Technology—AQUA 53, 8 (2004) 567-572.</ref><ref name="Zhao">{{cite journal|pmid=9450315|year=1998|last1=Zhao|first1=G|last2=Stevens Jr|first2=SE|title=Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion.|volume=11|issue=1|pages=27–32|journal=Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine}}</ref><ref name="Pedahzur">{{cite journal|doi=10.1016/S0273-1223(97)00240-0|title=Silver and hydrogen peroxide as potential drinking water disinfectants: their bactericidal effects and possible modes of action|year=1997|last1=Pedahzur|first1=R|journal=Water Science and Technology|volume=35|issue=11-12|pages=87}}</ref><ref name="Wuhrmann">Wuhrmann, Von K. and F. Zobrist. "Untersuchengen uber die bakterizide Wirkung von Silvber in Wasser." Schweizerische Zeitschrift fur Hydrologie 20 (1958) 218-255.</ref><ref name="Chambers">Chambers, Cecil W., Charles M. Proctor, and Paul W. Kabler. "Bactericidal Effect of Low Concentrations of Silver." Journal of the American Water Works Association 54 (1962) 208-216.</ref><ref name="Pedahzur2">{{cite journal|doi=10.1016/0273-1223(95)00252-I|title=The interaction of silver ions and hydrogen peroxide in the inactivation of E. coli: a preliminary evaluation of a new long acting residual drinking water disinfectant|year=1995|last1=Pedahzur|first1=R|journal=Water Science and Technology|volume=31|issue=5-6|pages=123}}</ref> | |||
| Much research has been done in evaluating the ability of the silver ion at inactivating '']'', a microorganism commonly used as an indicator for fecal contamination and as a surrogate for pathogens in drinking water treatment. Concentrations of silver nitrate evaluated in inactivation experiments range from 10–200 micrograms per liter as Ag<sup>+</sup>. | |||
| However, there are many inconsistencies in the literature regarding the kinetics of the inactivation of ''E. coli'' by the silver ion. With inconsistent data, it is impossible to tell what the true inactivation kinetics are, and therefore impossible to implement any sort of large-scale water treatment. | |||
| Silver's antimicrobial activity saw many applications prior to the discovery of modern antibiotics, when it fell into near disuse. Its association with ] made consumers wary and led them to turn away from it when given an alternative.{{cn|date=September 2023}} | |||
| The inconsistencies may be due to several factors. First, the kinetics may depend on the source of the silver ion being used. In recent years, research has focused largely on electrolytically generated silver ions or colloidal silver. Most studies in which the inactivation kinetics of ''E. coli'' by silver nitrate were explored extensively date back several decades. Even within this smaller group of studies, vast inconsistencies exist, likely due to inaccurate analytical methods for measuring the concentration of silver in solution.<ref name="Woodward">Woodward, Richard L. "Review of the Bactericidal Effectiveness of Silver." Journal of the American Water Works Association. 55.7 (1963) 881-886.</ref> Monitoring the decay of the silver ion in solution is imperative as silver tends to both adsorb readily to organic matter in the water and to be light reactive.<ref name="silver compounds">"Silver Compounds." Encyclopedia of Chemical Technology. Vol. 22. Fourth Ed. Excec. Ed. Jaqueline I. Kroschwitz. New York: John Wiley and Sons, 1997.</ref> Furthermore, silver tends to adsorb to glassware, which can lead not only to a decrease in the silver concentration within a given experiment but also to a release of the silver in subsequent experiments unless measures further than general glassware washing are taken for the removal of silver from the glassware surface.<ref name="Chambers" /> Therefore studies must both minimize the external factors affecting the concentration and to measure the changes in concentration that take place throughout the experiment. | |||
| ====Effects of various parameters==== | |||
| Despite the inconsistencies in the literature regarding the kinetics of the inactivation of ''E. coli'' by silver nitrate, important information can still be taken from the work. A study by Wuhrmann and Zobrist investigated the effect of various parameters upon the kinetics. First, they studied the effect of several ions in the water, including calcium, phosphates and chloride, all of which were found to decrease the bactericidal effect of silver.<ref name="Wuhrmann" /> These effects are important to consider when designing an experiment. Because of the effect of phosphates, it is undesirable to use phosphate buffer to run experiments, as this creates a phosphate concentration much higher than that found in natural waters and will falsely slow the inactivation kinetics. Furthermore, it is important to avoid touching any glassware with bare hands, as chloride from sweat may contaminate the glassware, again slowing inactivation. Chambers, Proctor and Kabler established the importance of using an effective neutralizer solution made of a combination of sodium thioglycolate and sodium thiosulfate, rather than sodium thiosulfate alone, which though it is effective in neutralizing other disinfectants does not sufficiently stop the bactericidal action of silver nitrate.<ref name="Chambers" /> Both tested the effect of pH on the kinetics, finding that a higher pH increased the bactericidal action.<ref name= "Chambers"/><ref name="silver compounds" /> Wuhrmann and Zobrist further established that at a higher temperature, inactivation occurs faster.<ref name="Wuhrmann" /> | |||
| ====Kinetic models==== | |||
| A further complication of the inactivation kinetics by silver is the question of which model to use. With most disinfectants, the inactivation is effectively modeled using a first-order Chick-Watson model, which states that a certain level of disinfection will occur at a certain ] (concentration * time).<ref name="Masters">Masters, Gilbert M. and Wendell P. Ela. "Introduction to Environmental Engineering and Science." Fifth Edition. Upper Saddle River: Prentice Hall, 2006.</ref> According to this model, the same amount of inactivation should take place when a concentration of 0.2 mg/L is applied for 10 minutes as when 0.02 mg/L is applied for 100 minutes. Wuhrmann and Zobrist found rate kinetics that followed this model for all conditions, which agrees fairly well with a study by Chambers and Proctor, while another study by Renn and Chesney found curves that did not follow this law.<ref name="Woodward" /> It is therefore unclear whether this law sufficiently models inactivation by the silver ion. | |||
| Most recent papers regarding the disinfection of ''E. coli'' by silver nitrate have simply plotted the level of disinfection against time.<ref name="Potapchenko" /><ref name="Kim" /><ref name="Matsumura" /><ref name="Zhao" /><ref name="Pedahzur" /> While this method of data analysis does not risk making false assumptions about first-order kinetics, it does nothing to account for the applied concentration, which is essential to any kinetics. Therefore, different curves need to be generated for each concentration that might be applied. Furthermore, it does not account for changes in concentration that might take place during the experiment, and which may vary based on many factors. | |||
| A third model which has been suggested for the inactivation kinetics by silver nitrate is that of Cs*T, or chemisorbed silver onto the cell body times time. This model suggests that the rate of inactivation depends not on the concentration in the water at a given time, but rather on the silver that has been chemisorbed by the bacteria. It is assumed, according to this model that C0 = C1 + C2 + C3, where C0 is the initial concentration, C1 is the silver still in solution, C2 is the silver lost to adsorption to glassware or other factors in the solution, and C3 is the silver chemisorbed to the bacteria. C0 is measured at the beginning of the experiment, C1 is measured throughout the experiment, and C2 is determine in a control experiment without bacteria. C3, or the Cs value, is then determined to be C0-C1-C2.<ref name="Hwang" /><ref name="Hwang2">{{cite journal|pmid=17037129|year=2006|last1=Hwang|first1=MG|last2=Katayama|first2=H|last3=Ohgaki|first3=S|title=Accumulation of copper and silver onto cell body and its effect on the inactivation of Pseudomonas aeruginosa.|volume=54|issue=3|pages=29–34|journal=Water science and technology : a journal of the International Association on Water Pollution Research}}</ref> According to Hwang, et al., this model was successful in estimating inactivation of ''E. coli'' by silver nitrate.<ref name="Hwang" /> Although it is possible that this model does not sufficiently account for all of the possible fates of the initial silver nitrate added to the solution, it is certainly a compelling method of data analysis. Because it is a new model, it has not been extensively studied by various researchers. | |||
| ===Against warts=== | ===Against warts=== | ||
| ] | |||
| Repeated daily application of silver nitrate can induce adequate destruction of cutaneous ], but occasionally pigmented scars may develop. In a placebo-controlled study of 70 | |||
| patients, silver nitrate given over nine days resulted in clearance of all warts in 43% and improvement in warts in 26% one month after treatment compared to 11% and 14%, respectively, in the placebo group.<ref> |
Repeated daily application of silver nitrate can induce adequate destruction of cutaneous ], but occasionally pigmented scars may develop. In a placebo-controlled study of 70 patients, silver nitrate given over nine days resulted in clearance of all warts in 43% and improvement in warts in 26% one month after treatment compared to 11% and 14%, respectively, in the placebo group.<ref>{{Cite journal | issue = 1 | volume = 144 | journal = British Journal of Dermatology | pages = 4–11 | year = 2001 | url = http://www.huidziekten.nl/richtlijnen/BADguidelineCutaneousWarts2001.pdf | pmid = 11167676 | doi = 10.1046/j.1365-2133.2001.04066.x | title = Guidelines for the management of cutaneous warts | first2 = S. | last2 = Handfield-Jones | last1 = Sterling | last3 = Hudson | author4 = British Association of Dermatologists | first1 = J. C. | first3 = P. M. | s2cid = 20179474 | url-status = dead | archive-url = https://web.archive.org/web/20120303031317/http://www.huidziekten.nl/richtlijnen/BADguidelineCutaneousWarts2001.pdf | archive-date = 2012-03-03 }}</ref> | ||
| ==Safety== | ==Safety== | ||
| As an oxidant, silver nitrate should be properly stored away from organic compounds. It reacts explosively with ethanol.<ref>{{Cite journal |last1=Perrin |first1=D. D. |last2=Armarego |first2=W. L. F. |last3=Perrin |first3=D. R. |date=November 1986 |title=Silver nitrate + ethanol = explosion |url=https://pubs.acs.org/doi/abs/10.1021/ed063p1016.1 |journal=Journal of Chemical Education |language=en |volume=63 |issue=11 |pages=1016 |doi=10.1021/ed063p1016.1 |bibcode=1986JChEd..63.1016P |issn=0021-9584}}</ref> Despite its common usage in extremely low concentrations to prevent ] and control nosebleeds, silver nitrate is still very toxic and corrosive.<ref>{{cite web|url=http://msds.chem.ox.ac.uk/SI/silver_nitrate.html|title=Safety data for silver nitrate (MSDS)|publisher=Oxford University Chemistry department|access-date=2008-03-25|archive-date=2011-12-02|archive-url=https://web.archive.org/web/20111202090543/http://msds.chem.ox.ac.uk/SI/silver_nitrate.html|url-status=dead}}</ref> Brief exposure will not produce any immediate side effects other than the purple, brown or black stains on the skin, but upon constant exposure to high concentrations, side effects will be noticeable, which include burns. Long-term exposure may cause eye damage. Silver nitrate is known to be a skin and eye irritant. Silver nitrate has not been thoroughly investigated for potential ].<ref>{{cite web|title= New Jersey Right-To-Know-Act Hazardous Substance Fact Sheet - Silver Nitrate|url=http://nj.gov/health/eoh/rtkweb/documents/fs/1672.pdf}}</ref> | |||
| {{Cleanup-rewrite|2=section|date=May 2009}} | |||
| ] | |||
| As an oxidant, silver nitrate should be properly stored away from organic compounds. Despite being used in low concentrations to prevent ] and control nose bleeds, silver nitrate is toxic and corrosive.<ref>{{cite web| url=http://msds.chem.ox.ac.uk/SI/silver_nitrate.html|title=Safety data for silver nitrate (MSDS)|publisher=Oxford University Chemistry department}}</ref> Brief exposure to the chemical will not produce immediate or even any side effects other than the purple, brown or black skin stains; but with more exposure, side effects will become more noticeable, including burns. Long-term exposure may cause eye damage. Short contact can lead to deposition of black silver stains on the skin. Besides being very destructive of mucous membranes, it is a skin and eye irritant. | |||
| Silver nitrate is currently unregulated in water sources by the United States Environmental Protection Agency. However, if more than 1 gram of silver is accumulated in the body, a condition called ] may develop. Argyria is a permanent cosmetic condition in which the skin and internal organs turn a blue-gray color. The United States Environmental Protection Agency used to have a maximum contaminant limit for silver in water until 1990, when it was determined that argyria did not impact the function of any affected organs despite the discolouration.<ref name="silver compounds">"Silver Compounds." Encyclopedia of Chemical Technology. Vol. 22. Fourth Ed. Excec. Ed. Jaqueline I. Kroschwitz. New York: John Wiley and Sons, 1997.</ref> Argyria is more often associated with the consumption of ] solutions rather than with silver nitrate, since it is only used at extremely low concentrations to disinfect the water. However, it is still important to be wary before ingesting any sort of silver-ion solution. | |||
| ==References== | ==References== | ||
| {{ |
{{reflist}} | ||
| ==External links== | ==External links== | ||
| {{Commons category}} | |||
| * | |||
| * | * | ||
| * | |||
| * | |||
| https://www.cofesilver.com/en/silver_bar :silver bar explanation. pricing investing | |||
| {{Silver compounds}} | {{Silver compounds}} | ||
| {{Nitrates}} | |||
| {{Antiseptics and disinfectants}} | |||
| {{Authority control}} | |||
| {{DEFAULTSORT:Silver Nitrate}} | |||
| ] | ] | ||
| ] | ] | ||
| Line 179: | Line 207: | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 07:15, 8 January 2025
 Structural formula | |

| |
 Crystal structure | |
| Names | |
|---|---|
| IUPAC name Silver nitrate | |
| Systematic IUPAC name Silver(I) nitrate | |
| Other names
Nitric acid silver(1+) salt Lapis infernalis Argentous nitrate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.958 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1493 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | AgNO3 |
| Molar mass | 169.872 g·mol |
| Appearance | colorless solid |
| Odor | Odorless |
| Density | 4.35 g/cm (24 °C) 3.97 g/cm (210 °C) |
| Melting point | 209.7 °C (409.5 °F; 482.8 K) |
| Boiling point | 440 °C (824 °F; 713 K) decomposes |
| Solubility in water | 122 g/100 mL (0 °C) 170 g/100 mL (10 °C) 256 g/100 mL (25 °C) 373 g/100 mL (40 °C) 912 g/100 mL (100 °C) |
| Solubility | Soluble in acetone, ammonia, ether, glycerol |
| Solubility in acetic acid | 0.776 g/kg (30 °C) 1.244 g/kg (40 °C) 5.503 g/kg (93 °C) |
| Solubility in acetone | 0.35 g/100 g (14 °C) 0.44 g/100 g (18 °C) |
| Solubility in benzene | 0.22 g/kg (35 °C) 0.44 g/kg (40.5 °C) |
| Solubility in ethanol | 3.1 g/100 g (19 °C) |
| Solubility in ethyl acetate | 2.7 g/100 g (20 °C) |
| log P | 0.19 |
| Magnetic susceptibility (χ) | −45.7·10 cm/mol |
| Refractive index (nD) | 1.744 |
| Viscosity | 3.77 cP (244 °C) 3.04 cP (275 °C) |
| Structure | |
| Crystal structure | Orthorhombic, oP56 |
| Space group | P212121, No. 19 |
| Point group | 222 |
| Lattice constant | a = 6.992(2) Å, b = 7.335(2) Å, c = 10.125(2) Åα = 90°, β = 90°, γ = 90° |
| Thermochemistry | |
| Heat capacity (C) | 93.1 J/mol·K |
| Std molar entropy (S298) |
140.9 J/mol·K |
| Std enthalpy of formation (ΔfH298) |
−124.4 kJ/mol |
| Gibbs free energy (ΔfG) | −33.4 kJ/mol |
| Pharmacology | |
| ATC code | D08AL01 (WHO) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Reacts explosively with ethanol. Toxic. Corrosive. |
| GHS labelling: | |
| Pictograms |    
|
| Signal word | Danger |
| Hazard statements | H272, H314, H410 |
| Precautionary statements | P220, P273, P280, P305+P351+P338, P310, P501 |
| NFPA 704 (fire diamond) |
 |
| Lethal dose or concentration (LD, LC): | |
| LDLo (lowest published) | 800 mg/kg (rabbit, oral) 20 mg/kg (dog, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Silver nitrate is an inorganic compound with chemical formula AgNO
3. It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called lunar caustic because silver was called luna by ancient alchemists who associated silver with the moon. In solid silver nitrate, the silver ions are three-coordinated in a trigonal planar arrangement.
Synthesis and structure
Albertus Magnus, in the 13th century, documented the ability of nitric acid to separate gold and silver by dissolving the silver. Indeed silver nitrate can be prepared by dissolving silver in nitric acid followed by evaporation of the solution. The stoichiometry of the reaction depends upon the concentration of nitric acid used.
- 3 Ag + 4 HNO3 (cold and diluted) → 3 AgNO3 + 2 H2O + NO
- Ag + 2 HNO3 (hot and concentrated) → AgNO3 + H2O + NO2
The structure of silver nitrate has been examined by X-ray crystallography several times. In the common orthorhombic form stable at ordinary temperature and pressure, the silver atoms form pairs with Ag---Ag contacts of 3.227 Å. Each Ag center is bonded to six oxygen centers of both uni- and bidentate nitrate ligands. The Ag-O distances range from 2.384 to 2.702 Å.
Reactions
A typical reaction with silver nitrate is to suspend a rod of copper in a solution of silver nitrate and leave it for a few hours. The silver nitrate reacts with copper to form hairlike crystals of silver metal and a blue solution of copper nitrate:
- 2 AgNO3 + Cu → Cu(NO3)2 + 2 Ag
Silver nitrate decomposes when heated:
Qualitatively, decomposition is negligible below the melting point, but becomes appreciable around 250 °C and fully decomposes at 440 °C.
Most metal nitrates thermally decompose to the respective oxides, but silver oxide decomposes at a lower temperature than silver nitrate, so the decomposition of silver nitrate yields elemental silver instead.
Uses
Precursor to other silver compounds
Silver nitrate is the least expensive salt of silver; it offers several other advantages as well. It is non-hygroscopic, in contrast to silver fluoroborate and silver perchlorate. In addition, it is relatively stable to light, and it dissolves in numerous solvents, including water. The nitrate can be easily replaced by other ligands, rendering AgNO3 versatile. Treatment with solutions of halide ions gives a precipitate of AgX (X = Cl, Br, I). When making photographic film, silver nitrate is treated with halide salts of sodium or potassium to form insoluble silver halide in situ in photographic gelatin, which is then applied to strips of tri-acetate or polyester. Similarly, silver nitrate is used to prepare some silver-based explosives, such as the fulminate, azide, or acetylide, through a precipitation reaction.
Treatment of silver nitrate with base gives dark grey silver oxide:
- 2 AgNO3 + 2 NaOH → Ag2O + 2 NaNO3 + H2O
Halide abstraction
The silver cation, Ag
, reacts quickly with halide sources to produce the insoluble silver halide, which is a cream precipitate if Br
is used, a white precipitate if Cl
is used and a yellow precipitate if I
is used. This reaction is commonly used in inorganic chemistry to abstract halides:
- Ag
(aq) + X
(aq) → AgX(s)
where X
= Cl
, Br
, or I
.
Other silver salts with non-coordinating anions, namely silver tetrafluoroborate and silver hexafluorophosphate are used for more demanding applications.
Similarly, this reaction is used in analytical chemistry to confirm the presence of chloride, bromide, or iodide ions. Samples are typically acidified with dilute nitric acid to remove interfering ions, e.g. carbonate ions and sulfide ions. This step avoids confusion of silver sulfide or silver carbonate precipitates with that of silver halides. The color of precipitate varies with the halide: white (silver chloride), pale yellow/cream (silver bromide), yellow (silver iodide). AgBr and especially AgI photo-decompose to the metal, as evidenced by a grayish color on exposed samples.
The same reaction was used on steamships in order to determine whether or not boiler feedwater had been contaminated with seawater. It is still used to determine if moisture on formerly dry cargo is a result of condensation from humid air, or from seawater leaking through the hull.
Organic synthesis
Silver nitrate is used in many ways in organic synthesis, e.g. for deprotection and oxidations. Ag
binds alkenes reversibly, and silver nitrate has been used to separate mixtures of alkenes by selective absorption. The resulting adduct can be decomposed with ammonia to release the free alkene. Silver nitrate is highly soluble in water but is poorly soluble in most organic solvents, except acetonitrile (111.8 g/100 g, 25 °C).
Biology
In histology, silver nitrate is used for silver staining, for demonstrating reticular fibers, proteins and nucleic acids. For this reason it is also used to demonstrate proteins in PAGE gels. It can be used as a stain in scanning electron microscopy.
Cut flower stems can be placed in a silver nitrate solution, which prevents the production of ethylene. This delays ageing of the flower.
Indelible ink
Silver nitrate produces long-lasting stain when applied to skin and is one of indelible ink’s ingredients. An electoral stain makes use of this to mark a finger of people who have voted in an election, allowing easy identification to prevent double-voting.
In addition to staining skin, silver nitrate has a history of use in stained glass. In the 14th century, artists began using a "silver stain" (also known as a yellow stain) made from silver nitrate to create a yellow effect on clear glass. The stain would produce a stable color that could range from pale lemon to deep orange or gold. Silver stain was often used with glass paint, and was applied to the opposite side of the glass as the paint. It was also used to create a mosaic effect by reducing the number of pieces of glass in a window. Despite the age of the technique, this process of creating stained glass remains almost entirely unchanged.
Medicine
See also: Medical uses of silver
Silver salts have antiseptic properties. In 1881 Credé introduced a method known as Credé's prophylaxis, which used of dilute (2%) solutions of silver nitrate in newborn babies' eyes at birth to prevent contraction of gonorrhea from the mother, which could cause blindness via ophthalmia neonatorum. (Modern antibiotics are now used instead).
Fused silver nitrate, shaped into sticks, was traditionally called "lunar caustic". It is used as a cauterizing agent, for example to remove granulation tissue around a stoma. General Sir James Abbott noted in his journals that in India in 1827 it was infused by a British surgeon into wounds in his arm resulting from the bite of a mad dog to cauterize the wounds and prevent the onset of rabies.
Silver nitrate is used to cauterize superficial blood vessels in the nose to help prevent nosebleeds.
Dentists sometimes use silver nitrate-infused swabs to heal oral ulcers. Silver nitrate is used by some podiatrists to kill cells located in the nail bed.
The Canadian physician C. A. Douglas Ringrose researched the use of silver nitrate for sterilization procedures, believing that silver nitrate could be used to block and corrode the fallopian tubes. The technique was ineffective.
Disinfection
Much research has been done in evaluating the ability of the silver ion at inactivating Escherichia coli, a microorganism commonly used as an indicator for fecal contamination and as a surrogate for pathogens in drinking water treatment. Concentrations of silver nitrate evaluated in inactivation experiments range from 10–200 micrograms per liter as Ag. Silver's antimicrobial activity saw many applications prior to the discovery of modern antibiotics, when it fell into near disuse. Its association with argyria made consumers wary and led them to turn away from it when given an alternative.
Against warts

Repeated daily application of silver nitrate can induce adequate destruction of cutaneous warts, but occasionally pigmented scars may develop. In a placebo-controlled study of 70 patients, silver nitrate given over nine days resulted in clearance of all warts in 43% and improvement in warts in 26% one month after treatment compared to 11% and 14%, respectively, in the placebo group.
Safety
As an oxidant, silver nitrate should be properly stored away from organic compounds. It reacts explosively with ethanol. Despite its common usage in extremely low concentrations to prevent gonorrhea and control nosebleeds, silver nitrate is still very toxic and corrosive. Brief exposure will not produce any immediate side effects other than the purple, brown or black stains on the skin, but upon constant exposure to high concentrations, side effects will be noticeable, which include burns. Long-term exposure may cause eye damage. Silver nitrate is known to be a skin and eye irritant. Silver nitrate has not been thoroughly investigated for potential carcinogenic effect.
Silver nitrate is currently unregulated in water sources by the United States Environmental Protection Agency. However, if more than 1 gram of silver is accumulated in the body, a condition called argyria may develop. Argyria is a permanent cosmetic condition in which the skin and internal organs turn a blue-gray color. The United States Environmental Protection Agency used to have a maximum contaminant limit for silver in water until 1990, when it was determined that argyria did not impact the function of any affected organs despite the discolouration. Argyria is more often associated with the consumption of colloidal silver solutions rather than with silver nitrate, since it is only used at extremely low concentrations to disinfect the water. However, it is still important to be wary before ingesting any sort of silver-ion solution.
References
- ^ Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York City: D. Van Nostrand Company. pp. 617–619.
- ^ Kiper, Ruslan Anatolievich. "silver nitrate". Chemister.ru. Retrieved 2014-07-20.
- ^ Meyer, P.; Rimsky, A.; Chevalier, R. (1978). "Structure du nitrate d'argent à pression et température ordinaires. Exemple de cristal parfait". Acta Crystallogr. B. 34 (5): 1457–1462. Bibcode:1978AcCrB..34.1457M. doi:10.1107/S0567740878005907.
- ^ Sigma-Aldrich Co., Silver nitrate. Retrieved on 2014-07-20.
- "Silver (metal dust and soluble compounds, as Ag)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "Definition of Lunar Caustic". dictionary.die.net. Archived from the original on 2012-01-31.
- Szabadváry, Ferenc (1992). History of analytical chemistry. Taylor & Francis. p. 17. ISBN 978-2-88124-569-5.
- Stern, K. H. (1972). "High Temperature Properties and Decomposition of Inorganic Salts Part 3, Nitrates and Nitrites". Journal of Physical and Chemical Reference Data. 1 (3): 747–772. Bibcode:1972JPCRD...1..747S. doi:10.1063/1.3253104. S2CID 95532988.
- Campaigne, E.; LeSuer, W. M. (1963). "3-Thiophenecarboxylic (Thenoic) Acid". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 4, p. 919. (preparation of Ag2O, used in oxidation of an aldehyde) - "Silver nitrate method". Transport Information Service. Gesamtverband der Deutschen Versicherungswirtschaf. Retrieved 22 June 2015.
- Cope, A. C.; Bach, R. D. (1973). "trans-Cyclooctene". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 5, p. 315. - "silver nitrate". chemister.ru. Retrieved 2019-04-04.
- Geissinger HD (2011). "The use of silver nitrate as a stain for scanning electron microscopy of arterial intima and paraffin sections of kidney". Journal of Microscopy. 95 (3): 471–481. doi:10.1111/j.1365-2818.1972.tb01051.x. PMID 4114959. S2CID 38335416.
- "Silver Nitrate (072503) Fact Sheet" (PDF). epa.gov. Retrieved 3 October 2024.
- Dhillon, Amrit (2023-06-17). "The ink with a 'secret formula' that powers the world's biggest democratic exercise | India | The Guardian". The Guardian. Archived from the original on 2023-06-17. Retrieved 2024-04-17.
- Dhillon, Amrit (2019-03-28). "The ink with a 'secret formula' that powers the world's biggest democratic exercise". The Guardian. ISSN 0261-3077. Retrieved 2024-04-17.
- "Khan Academy". www.khanacademy.org. Retrieved 2024-10-29.
- Matejcek, A; Goldman, RD (November 2013). "Treatment and prevention of ophthalmia neonatorum". Canadian Family Physician. 59 (11): 1187–90. PMC 3828094. PMID 24235191.
- Peter.H (2000). "Dr Carl Credé (1819–1892) and the prevention of ophthalmia neonatorum". Arch Dis Child Fetal Neonatal Ed. 83 (2): F158 – F159. doi:10.1136/fn.83.2.F158. PMC 1721147. PMID 10952715.
- Credé C. S. E. (1881). "Die Verhürtung der Augenentzündung der Neugeborenen". Archiv für Gynäkologie. 17 (1): 50–53. doi:10.1007/BF01977793. S2CID 10053605.
- Schaller, Ulrich C. & Klauss, Volker (2001). "Is Credés prophylaxis for ophthalmia neonatorum still valid?". Bulletin of the World Health Organization. 79 (3): 262–266. PMC 2566367. PMID 11285676.
- British Library, India Office Records, European Manuscripts, MSS EUR F171/33/3, page 109.
- Ringrose CA. (1973). "Office tubal sterilization". Obstetrics and Gynecology. 42 (1): 151–5. PMID 4720201.
- Cryderman v. Ringrose (1978), 89 D.L.R. (3d) 32 (Alta S.C.) and Zimmer et al. v. Ringrose (1981) 4 W.W.R. 75 (Alta C.A.).
- Sterling, J. C.; Handfield-Jones, S.; Hudson, P. M.; British Association of Dermatologists (2001). "Guidelines for the management of cutaneous warts" (PDF). British Journal of Dermatology. 144 (1): 4–11. doi:10.1046/j.1365-2133.2001.04066.x. PMID 11167676. S2CID 20179474. Archived from the original (PDF) on 2012-03-03.
- Perrin, D. D.; Armarego, W. L. F.; Perrin, D. R. (November 1986). "Silver nitrate + ethanol = explosion". Journal of Chemical Education. 63 (11): 1016. Bibcode:1986JChEd..63.1016P. doi:10.1021/ed063p1016.1. ISSN 0021-9584.
- "Safety data for silver nitrate (MSDS)". Oxford University Chemistry department. Archived from the original on 2011-12-02. Retrieved 2008-03-25.
- "New Jersey Right-To-Know-Act Hazardous Substance Fact Sheet - Silver Nitrate" (PDF).
- "Silver Compounds." Encyclopedia of Chemical Technology. Vol. 22. Fourth Ed. Excec. Ed. Jaqueline I. Kroschwitz. New York: John Wiley and Sons, 1997.
External links
- International Chemical Safety Card 1116
- NIOSH Pocket Guide to Chemical Hazards
- History of Kodak: About Film and Imaging
https://www.cofesilver.com/en/silver_bar :silver bar explanation. pricing investing
| Silver compounds | |||
|---|---|---|---|
| Silver(0,I) | |||
| Silver(I) |
| ||
| Silver(II) | |||
| Silver(III) | |||
| Silver(I,III) | |||
| Salts and covalent derivatives of the nitrate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antiseptics and disinfectants (D08) | |
|---|---|
| Acridine derivatives | |
| Biguanides and amidines | |
| Phenol and derivatives | |
| Nitrofuran derivatives | |
| Iodine products | |
| Quinoline derivatives | |
| Quaternary ammonium compounds | |
| Mercurial products | |
| Silver compounds | |
| Alcohols | |
| Other | |
| |
