| Revision as of 21:20, 9 March 2016 editFonsy74 (talk | contribs)77 edits Target receptor information with sources.Tag: Visual edit← Previous edit | Latest revision as of 19:21, 6 August 2024 edit undoMarbletan (talk | contribs)Extended confirmed users5,491 editsm →Medical use | ||
| (114 intermediate revisions by 38 users not shown) | |||
| Line 2: | Line 2: | ||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = 415530375 | | verifiedrevid = 415530375 | ||
| | Reference =<ref> |
| Reference = <ref>{{cite web | url = http://www.sigmaaldrich.com/catalog/search/ProductDetail/ALDRICH/A76109 | title = Homotaurine | publisher = ]}}</ref> | ||
| | ImageFile_Ref = {{chemboximage|correct|??}} | | ImageFile_Ref = {{chemboximage|correct|??}} | ||
| | ImageFile = Homotaurine.svg | | ImageFile = Homotaurine.svg | ||
| Line 9: | Line 9: | ||
| | ImageFile1 = Homotaurine-3D-balls.png | | ImageFile1 = Homotaurine-3D-balls.png | ||
| | ImageAlt1 = Ball-and-stick model | | ImageAlt1 = Ball-and-stick model | ||
| | |
| PIN = 3-Aminopropane-1-sulfonic acid | ||
| | OtherNames =Tramiprosate; Alzhemed; 3-APS | | OtherNames = Tramiprosate; Alzhemed; 3-APS | ||
| |Section1={{Chembox Identifiers | |Section1={{Chembox Identifiers | ||
| ⚫ | | CASNo_Ref = {{cascite|correct|??}} | ||
| ⚫ | | CASNo = 3687-18-1 | ||
| | ChEBI = 1457 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 1584 | | ChemSpiderID = 1584 | ||
| ⚫ | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| ⚫ | | ChEMBL = 149082 | ||
| | DrugBank = DB06527 | |||
| | EC_number = 222-977-4 | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = D06202 | | KEGG = D06202 | ||
| ⚫ | | PubChem = 1646 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 5K8EAX0G53 | |||
| | InChI = 1/C3H9NO3S/c4-2-1-3-8(5,6)7/h1-4H2,(H,5,6,7) | | InChI = 1/C3H9NO3S/c4-2-1-3-8(5,6)7/h1-4H2,(H,5,6,7) | ||
| | InChIKey = SNKZJIOFVMKAOJ-UHFFFAOYAT | | InChIKey = SNKZJIOFVMKAOJ-UHFFFAOYAT | ||
| ⚫ | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| ⚫ | | ChEMBL = 149082 | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C3H9NO3S/c4-2-1-3-8(5,6)7/h1-4H2,(H,5,6,7) | | StdInChI = 1S/C3H9NO3S/c4-2-1-3-8(5,6)7/h1-4H2,(H,5,6,7) | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = SNKZJIOFVMKAOJ-UHFFFAOYSA-N | | StdInChIKey = SNKZJIOFVMKAOJ-UHFFFAOYSA-N | ||
| ⚫ | | CASNo_Ref = {{cascite|correct|??}} | ||
| ⚫ | | CASNo =3687-18-1 | ||
| ⚫ | | PubChem =1646 | ||
| | SMILES = O=S(=O)(O)CCCN | | SMILES = O=S(=O)(O)CCCN | ||
| }} | }} | ||
| |Section2={{Chembox Properties | |Section2={{Chembox Properties | ||
| | C=3 | H=9 | N=1 | O=3 | S=1 | | C=3 | H=9 | N=1 | O=3 | S=1 | ||
| Line 42: | Line 47: | ||
| | FlashPt = | | FlashPt = | ||
| | AutoignitionPt = | | AutoignitionPt = | ||
| | GHS_ref=<ref>{{cite web |title=Tramiprosate |url=https://pubchem.ncbi.nlm.nih.gov/compound/1646#section=Safety-and-Hazards |website=pubchem.ncbi.nlm.nih.gov |access-date=13 December 2021 |language=en}}</ref> | |||
| | RPhrases ={{R36/37/38}} | |||
| | |
| GHSPictograms = {{GHS07}} | ||
| | GHSSignalWord = Warning | |||
| | HPhrases = {{H-phrases|315|319|335}} | |||
| | PPhrases = {{P-phrases|261|264|271|280|302+352|304+340|305+351+338|312|321|332+313|337+313|362|403+233|405|501}} | |||
| }} | }} | ||
| }} | }} | ||
| '''Homotaurine''' (also known as '''tramiprosate''' (]), '''3-amino-1-propanesulfonic acid''', or '''3-APS''') is a natural sulfonic acid found in seaweed.<ref>{{cite journal |last1=Martorana |first1=Alessandro |last2=Di Lorenzo |first2=Francesco |last3=Manenti |first3=Guglielmo |last4=Semprini |first4=Roberta |last5=Koch |first5=Giacomo |title=Homotaurine Induces Measurable Changes of Short Latency Afferent Inhibition in a Group of Mild Cognitive Impairment Individuals |journal=Frontiers in Aging Neuroscience |date=23 September 2014 |volume=6 |page=254 |doi=10.3389/fnagi.2014.00254 |pmid=25295005 |pmc=4172065 |doi-access=free }}</ref> It is analogous to ], but with an extra carbon in its chain. It has ] activity, apparently by mimicking GABA, which it resembles.<ref name=OrgChem2007/> | |||
| '''Homotaurine''' ('''3-amino-1-propanesulfonic acid''' ('''3-APS''') or '''tramiprosate''' (])) is a synthetic ]. It is analogous to ], but with an extra carbon in its chain. Because of its similarity in structure to the ] ] (GABA), it has ] effects and may be useful as an anticonvulsant.<ref>{{cite journal |author=Fariello RG, Golden GT, Pisa M |title=Homotaurine (3 aminopropanesulfonic acid; 3APS) protects from the convulsant and cytotoxic effect of systemically administered kainic acid |journal=Neurology |volume=32 |issue=3 |pages=241–5 |year=1982 |pmid=7199633 |doi=10.1212/wnl.32.3.241|last2=Golden |last3=Pisa }}</ref> | |||
| Homotaurine was investigated in a ] clinical trial as a potential treatment for ] (AD) that did not show efficacy. However, post-hoc analyses have shown positive and significant effects of homotaurine on secondary endpoints and subgroups of patients, including a reduction in hippocampal volume loss and lower decline in memory function in the overall cohort, as well as a reduction in global cognitive decline in APOE4 allele carriers, suggesting a ].<ref name=AD2012rev>{{cite journal |last1=Caltagirone |first1=C |last2=Ferrannini |first2=L |last3=Marchionni |first3=N |last4=Nappi |first4=G |last5=Scapagnini |first5=G |last6=Trabucchi |first6=M |title=The potential protective effect of tramiprosate (homotaurine) against Alzheimer's disease: a review |journal=Aging Clinical and Experimental Research |date=December 2012 |volume=24 |issue=6 |pages=580–587 |doi=10.3275/8585 |pmid=22961121 |s2cid=10816430 }}</ref> A study in cognitive impairment done in 2018 did show positive benefits.<ref>{{cite journal |last1=Martorana |first1=A. |last2=Motta |first2=C |last3=Koch |first3=G. |last4=Massaia |first4=M. |last5=Mondino |first5=S. |last6=Raniero |first6=I. |last7=Vacca |first7=A. |last8=Di Lorenzo |first8=F. |last9=Cavallo |first9=G. |last10=Oddenino |first10=E. |last11=Pavanelli |first11=E. |last12=Maniscalco |first12=M. |last13=Montano |first13=V. |last14=Mastropietro |first14=A. |last15=Bellia |first15=N. C. |last16=Ciravegna |first16=E. |last17=La Rocca |first17=M. |last18=Vitale |first18=E. |last19=Lorico |first19=F. |last20=Zacchettin |first20=B. |last21=Scalise |first21=A. |last22=Codemo |first22=A. |last23=Gabelli |first23=C. |last24=Spano |first24=M. |last25=Poli |first25=S. |last26=Panuccio |first26=D. |last27=Bruno |first27=P. |last28=Alfieri |first28=P. |last29=Ruggiero |first29=R. |last30=Cursi |first30=F. |last31=Levi Della Vida |first31=G. |title=Effect of homotaurine in patients with cognitive impairment: results from an Italian observational retrospective study |journal=Journal of Gerontology and Geriatrics |date=15 March 2018 |volume=66 |pages=15–20 |url=http://www.jgerontology-geriatrics.com/article/view/97 }}</ref> | |||
| It is a ] receptor antagonist <ref>{{cite journal|title=Homotaurine: a GABAB antagonist in guinea-pig ileum.|pmid=6652358|author=Giotti A, Luzzi S, Spagnesi S, Zilletti L.}}</ref> and reversed the catatonia induced by baclofen in rats. <ref>{{cite journal|title=Baclofen induces catatonia in rats.|pmid=2823166|author=Mehta AK1, Ticku MK.}}</ref>Moreover, Homotaurine suppress ethanol-stimulated dopamine release, ethanol intake and preference in rats similar to ] (Acetylated homotaurine analogue) <ref>{{cite journal|title=Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release.|pmid=11864639|author=Olive MF, Nannini MA, Ou CJ, Koenig HN, Hodge CW.}}</ref>. | |||
| Homotaurine is currently in a phase 3 study with expected FDA approval as the first disease modifying drug for AD.<ref name="Tolar et al 2020">{{cite journal |last1=Tolar |first1=Martin |last2=Abushakra |first2=Susan |last3=Hey |first3=John A. |last4=Porsteinsson |first4=Anton |last5=Sabbagh |first5=Marwan |title=Aducanumab, gantenerumab, BAN2401, and ALZ-801—the first wave of amyloid-targeting drugs for Alzheimer's disease with potential for near term approval |journal=Alzheimer's Research & Therapy |date=December 2020 |volume=12 |issue=1 |pages=95 |doi=10.1186/s13195-020-00663-w |pmid=32787971 |pmc=7424995 |doi-access=free }}</ref><ref>{{cite journal |last1=Abushakra |first1=S. |last2=Porsteinsson |first2=A. |last3=Scheltens |first3=P. |last4=Sadowsky |first4=C. |last5=Vellas |first5=B. |last6=Cummings |first6=J. |last7=Gauthier |first7=S. |last8=Hey |first8=J. A. |last9=Power |first9=A. |last10=Wang |first10=P. |last11=Tolar |first11=M. |last12=Tolar |first12=M |title=Clinical effects of tramiprosate in apoe4/4 homozygous patients with mild alzheimer's disease suggest disease modification potential |journal=Journal of Prevention of Alzheimer's Disease |date=1 September 2017 |volume=4 |issue=3 |pages=149–156 |doi=10.14283/jpad.2017.26 |pmid=29182706 |s2cid=44515548 |doi-access=free }}</ref> | |||
| Homotaurine is a ] at neutral ]. | |||
| ==Medical use== | |||
| {{multiple image | |||
| ] (''N''-acetyl homotaurine) was approved by the FDA in 2004 to treat ].<ref name=OrgChem2007>{{cite book | last1 = Lednicer | first1 = Daniel | name-list-style = vanc | title = The Organic Chemistry of Drug Synthesis | date = 2008 | publisher = John Wiley & Sons | location = Hoboken | isbn = 978-0-470-18066-2 | edition = 7th | url = https://books.google.com/books?id=N6OAhuiHqiIC&pg=PA15 | page = 15 }}</ref> | |||
| | align = left | |||
| | direction = vertical | |||
| | footer = The ]ic form of homotaurine| width = 200 | |||
| | image1 = Aminopropanesulfonic acid zwitterionic.png | |||
| | alt1 = Skeletal formula | |||
| | image2 = Homotaurine-zwitterion-3D-balls.png | |||
| | alt2 = Ball-and-stick model | |||
| }} | |||
| ==Biochemical properties== | |||
| {{clear-left}} | |||
| In preclinical studies it had been found to bind to soluble ] and inhibit the formation of neurotoxic aggregates.<ref name=AD2012rev/><ref name="pmid17908052">{{cite journal |last1=Aisen |first1=Paul |last2=Gauthier |first2=Serge |last3=Vellas |first3=Bruno |last4=Briand |first4=Richard |last5=Saumier |first5=Daniel |last6=Laurin |first6=Julie |last7=Garceau |first7=Denis |title=Alzhemed: A Potential Treatment for Alzheimers Disease |journal=Current Alzheimer Research |date=1 September 2007 |volume=4 |issue=4 |pages=473–478 |doi=10.2174/156720507781788882 |pmid=17908052 }}</ref> Homotaurine has also shown ] activities, reduction in skeletal ], and ] activity.<ref name=Metabolism2013/> | |||
| Homotaurine has been reported as a ] antagonist,<ref name=OrgChem2007/> as well as a GABA agonist.<ref name=Metabolism2013>{{cite book |last1=Lajtha |first1=Abel |title=Metabolism in the Nervous System |date=2013 |publisher=Springer Science & Business Media |isbn=978-1-4684-4367-7 |page=520 |url=https://books.google.com/books?id=du_TBwAAQBAJ&pg=PA520 }}</ref><ref name=PharmPrinc2011>{{cite book |last1=Tashjian |first1=Armen H. |last2=Armstrong |first2=Ehrin J. |title=Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy |date=2011 |publisher=Lippincott Williams & Wilkins |isbn=978-1-4511-1805-6 |page=308 |url=https://books.google.com/books?id=kjCCMZHInigC&pg=PA308 }}</ref> '']'' studies have found that homotaurine is a ] partial agonist<ref>{{cite journal |last1=Reyes-Haro |first1=Daniel |last2=Cabrera-Ruíz |first2=Elizabeth |last3=Estrada-Mondragón |first3=Argel |last4=Miledi |first4=Ricardo |last5=Martínez-Torres |first5=Ataúlfo |title=Modulation of GABA-A receptors of astrocytes and STC-1 cells by taurine structural analogs |journal=Amino Acids |date=November 2014 |volume=46 |issue=11 |pages=2587–2593 |doi=10.1007/s00726-014-1813-0 |pmid=25119985 |s2cid=10319072 }}</ref> as well as a ] receptor partial agonist with low efficacy, becoming an antagonist and displacing the full agonists GABA and ] at this receptor.<ref>{{cite journal |last1=Giotti |first1=A. |last2=Luzzi |first2=S. |last3=Spagnesi |first3=S. |last4=Zilletti |first4=L. |title=Homotaurine: a GABAB antagonist in guinea-pig ileum. |journal=British Journal of Pharmacology |date=August 1983 |volume=79 |issue=4 |pages=855–862 |doi=10.1111/j.1476-5381.1983.tb10529.x |pmid=6652358 |pmc=2044932 }}</ref> In a study in rats, homotaurine reversed the ] induced by ] (the prototypical GABA<sub>B</sub> agonist),<ref>{{cite journal |last1=Mehta |first1=A |last2=Ticku |first2=M |title=Baclofen induces catatonia in rats |journal=Neuropharmacology |date=September 1987 |volume=26 |issue=9 |pages=1419–1423 |doi=10.1016/0028-3908(87)90108-0 |pmid=2823166 |s2cid=24010833 }}</ref> and was able to produce analgesia via the GABA<sub>B</sub> receptor, an effect that was abolished when ], a GABA<sub>B</sub> receptor antagonist was applied.<ref>{{cite journal |last1=Serrano |first1=M.Isabel |last2=Serrano |first2=Jose S. |last3=Fernández |first3=Ana |last4=Asadi |first4=Ihklas |last5=Serrano-Martino |first5=M.Carmen |title=GABAB Receptors and Opioid Mechanisms Involved in Homotaurine-Induced Analgesia |journal=General Pharmacology: The Vascular System |date=March 1998 |volume=30 |issue=3 |pages=411–415 |doi=10.1016/s0306-3623(97)00279-6 |pmid=9510095 }}</ref><ref>{{cite journal |last1=Serrano |first1=Maria Isabel |last2=Serrano |first2=Jose S. |last3=Asadi |first3=Ikhlas |last4=Fernandez |first4=Ana |last5=Serrano-Martino |first5=Maria Carmen |title=Role of K+-channels in homotaurine-induced analgesia |journal=Fundamental and Clinical Pharmacology |date=16 June 2001 |volume=15 |issue=3 |pages=167–173 |doi=10.1046/j.1472-8206.2001.00026.x |pmid=11468027 |s2cid=19694376 }}</ref> | |||
| ==See also== | |||
| * ] | |||
| In a human study homotaurine selectively and fully inhibits the formation of Aβ42 oligomers at the clinical dose, without evidence of vasogenic edema.<ref name="Tolar et al 2020"/> | |||
| ⚫ | ==References== | ||
| ⚫ | {{Reflist}} | ||
| One study in rats showed that homotaurine suppressed ethanol-stimulated dopamine release, as well as ethanol intake and preference in rats in a way similar to the ''N''-acetyl ] of homotaurine, ].<ref>{{cite journal |last1=Olive |first1=M.Foster |last2=Nannini |first2=Michelle A |last3=Ou |first3=Christine J |last4=Koenig |first4=Heather N |last5=Hodge |first5=Clyde W |title=Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release |journal=European Journal of Pharmacology |date=February 2002 |volume=437 |issue=1–2 |pages=55–61 |doi=10.1016/s0014-2999(02)01272-4 |pmid=11864639 }}</ref> | |||
| ⚫ | == References == | ||
| ⚫ | {{Reflist|33em}} | ||
| {{Anticonvulsants}} | {{Anticonvulsants}} | ||
| Line 81: | Line 85: | ||
| ] | ] | ||
| ] | ] | ||
| {{nervous-system-drug-stub}} | |||
Latest revision as of 19:21, 6 August 2024
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 3-Aminopropane-1-sulfonic acid | |
| Other names Tramiprosate; Alzhemed; 3-APS | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.020.889 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H9NO3S |
| Molar mass | 139.17 g·mol |
| Melting point | 293 °C (559 °F; 566 K) (decomposition) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
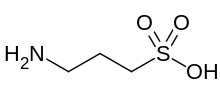
Homotaurine (also known as tramiprosate (INN), 3-amino-1-propanesulfonic acid, or 3-APS) is a natural sulfonic acid found in seaweed. It is analogous to taurine, but with an extra carbon in its chain. It has GABAergic activity, apparently by mimicking GABA, which it resembles.
Homotaurine was investigated in a Phase III clinical trial as a potential treatment for Alzheimer's disease (AD) that did not show efficacy. However, post-hoc analyses have shown positive and significant effects of homotaurine on secondary endpoints and subgroups of patients, including a reduction in hippocampal volume loss and lower decline in memory function in the overall cohort, as well as a reduction in global cognitive decline in APOE4 allele carriers, suggesting a disease-modifying effect. A study in cognitive impairment done in 2018 did show positive benefits.
Homotaurine is currently in a phase 3 study with expected FDA approval as the first disease modifying drug for AD.
Medical use
Acamprosate (N-acetyl homotaurine) was approved by the FDA in 2004 to treat alcohol dependence.
Biochemical properties
In preclinical studies it had been found to bind to soluble amyloid beta and inhibit the formation of neurotoxic aggregates. Homotaurine has also shown anticonvulsant activities, reduction in skeletal muscle tonus, and hypothermic activity.
Homotaurine has been reported as a GABA antagonist, as well as a GABA agonist. In vitro studies have found that homotaurine is a GABAA partial agonist as well as a GABAB receptor partial agonist with low efficacy, becoming an antagonist and displacing the full agonists GABA and baclofen at this receptor. In a study in rats, homotaurine reversed the catatonia induced by baclofen (the prototypical GABAB agonist), and was able to produce analgesia via the GABAB receptor, an effect that was abolished when CGP-35348, a GABAB receptor antagonist was applied.
In a human study homotaurine selectively and fully inhibits the formation of Aβ42 oligomers at the clinical dose, without evidence of vasogenic edema.
One study in rats showed that homotaurine suppressed ethanol-stimulated dopamine release, as well as ethanol intake and preference in rats in a way similar to the N-acetyl derivative of homotaurine, acamprosate.
References
- "Homotaurine". Sigma-Aldrich.
- "Tramiprosate". pubchem.ncbi.nlm.nih.gov. Retrieved 13 December 2021.
- Martorana, Alessandro; Di Lorenzo, Francesco; Manenti, Guglielmo; Semprini, Roberta; Koch, Giacomo (23 September 2014). "Homotaurine Induces Measurable Changes of Short Latency Afferent Inhibition in a Group of Mild Cognitive Impairment Individuals". Frontiers in Aging Neuroscience. 6: 254. doi:10.3389/fnagi.2014.00254. PMC 4172065. PMID 25295005.
- ^ Lednicer D (2008). The Organic Chemistry of Drug Synthesis (7th ed.). Hoboken: John Wiley & Sons. p. 15. ISBN 978-0-470-18066-2.
- ^ Caltagirone, C; Ferrannini, L; Marchionni, N; Nappi, G; Scapagnini, G; Trabucchi, M (December 2012). "The potential protective effect of tramiprosate (homotaurine) against Alzheimer's disease: a review". Aging Clinical and Experimental Research. 24 (6): 580–587. doi:10.3275/8585. PMID 22961121. S2CID 10816430.
- Martorana, A.; Motta, C; Koch, G.; Massaia, M.; Mondino, S.; Raniero, I.; Vacca, A.; Di Lorenzo, F.; Cavallo, G.; Oddenino, E.; Pavanelli, E.; Maniscalco, M.; Montano, V.; Mastropietro, A.; Bellia, N. C.; Ciravegna, E.; La Rocca, M.; Vitale, E.; Lorico, F.; Zacchettin, B.; Scalise, A.; Codemo, A.; Gabelli, C.; Spano, M.; Poli, S.; Panuccio, D.; Bruno, P.; Alfieri, P.; Ruggiero, R.; Cursi, F.; Levi Della Vida, G. (15 March 2018). "Effect of homotaurine in patients with cognitive impairment: results from an Italian observational retrospective study". Journal of Gerontology and Geriatrics. 66: 15–20.
- ^ Tolar, Martin; Abushakra, Susan; Hey, John A.; Porsteinsson, Anton; Sabbagh, Marwan (December 2020). "Aducanumab, gantenerumab, BAN2401, and ALZ-801—the first wave of amyloid-targeting drugs for Alzheimer's disease with potential for near term approval". Alzheimer's Research & Therapy. 12 (1): 95. doi:10.1186/s13195-020-00663-w. PMC 7424995. PMID 32787971.
- Abushakra, S.; Porsteinsson, A.; Scheltens, P.; Sadowsky, C.; Vellas, B.; Cummings, J.; Gauthier, S.; Hey, J. A.; Power, A.; Wang, P.; Tolar, M.; Tolar, M (1 September 2017). "Clinical effects of tramiprosate in apoe4/4 homozygous patients with mild alzheimer's disease suggest disease modification potential". Journal of Prevention of Alzheimer's Disease. 4 (3): 149–156. doi:10.14283/jpad.2017.26. PMID 29182706. S2CID 44515548.
- Aisen, Paul; Gauthier, Serge; Vellas, Bruno; Briand, Richard; Saumier, Daniel; Laurin, Julie; Garceau, Denis (1 September 2007). "Alzhemed: A Potential Treatment for Alzheimers Disease". Current Alzheimer Research. 4 (4): 473–478. doi:10.2174/156720507781788882. PMID 17908052.
- ^ Lajtha, Abel (2013). Metabolism in the Nervous System. Springer Science & Business Media. p. 520. ISBN 978-1-4684-4367-7.
- Tashjian, Armen H.; Armstrong, Ehrin J. (2011). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. p. 308. ISBN 978-1-4511-1805-6.
- Reyes-Haro, Daniel; Cabrera-Ruíz, Elizabeth; Estrada-Mondragón, Argel; Miledi, Ricardo; Martínez-Torres, Ataúlfo (November 2014). "Modulation of GABA-A receptors of astrocytes and STC-1 cells by taurine structural analogs". Amino Acids. 46 (11): 2587–2593. doi:10.1007/s00726-014-1813-0. PMID 25119985. S2CID 10319072.
- Giotti, A.; Luzzi, S.; Spagnesi, S.; Zilletti, L. (August 1983). "Homotaurine: a GABAB antagonist in guinea-pig ileum". British Journal of Pharmacology. 79 (4): 855–862. doi:10.1111/j.1476-5381.1983.tb10529.x. PMC 2044932. PMID 6652358.
- Mehta, A; Ticku, M (September 1987). "Baclofen induces catatonia in rats". Neuropharmacology. 26 (9): 1419–1423. doi:10.1016/0028-3908(87)90108-0. PMID 2823166. S2CID 24010833.
- Serrano, M.Isabel; Serrano, Jose S.; Fernández, Ana; Asadi, Ihklas; Serrano-Martino, M.Carmen (March 1998). "GABAB Receptors and Opioid Mechanisms Involved in Homotaurine-Induced Analgesia". General Pharmacology: The Vascular System. 30 (3): 411–415. doi:10.1016/s0306-3623(97)00279-6. PMID 9510095.
- Serrano, Maria Isabel; Serrano, Jose S.; Asadi, Ikhlas; Fernandez, Ana; Serrano-Martino, Maria Carmen (16 June 2001). "Role of K+-channels in homotaurine-induced analgesia". Fundamental and Clinical Pharmacology. 15 (3): 167–173. doi:10.1046/j.1472-8206.2001.00026.x. PMID 11468027. S2CID 19694376.
- Olive, M.Foster; Nannini, Michelle A; Ou, Christine J; Koenig, Heather N; Hodge, Clyde W (February 2002). "Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release". European Journal of Pharmacology. 437 (1–2): 55–61. doi:10.1016/s0014-2999(02)01272-4. PMID 11864639.
| GABA receptor modulators | |||||
|---|---|---|---|---|---|
| Ionotropic |
| ||||
| Metabotropic |
| ||||