| Revision as of 01:04, 29 January 2023 editEntranced98 (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers173,243 edits Importing Wikidata short description: "Pharmaceutical drug"Tag: Shortdesc helper← Previous edit | Revision as of 21:15, 22 October 2023 edit undoGabbyTheDarkAlien (talk | contribs)Extended confirmed users752 editsm removed unnecessary commaTag: Visual editNext edit → | ||
| Line 61: | Line 61: | ||
| == Medical uses == | == Medical uses == | ||
| Cevimeline is used in the treatment of ] (dry mouth) |
Cevimeline is used in the treatment of ] (dry mouth)<ref name="Ono_2004">{{cite journal | vauthors = Ono M, Takamura E, Shinozaki K, Tsumura T, Hamano T, Yagi Y, Tsubota K | title = Therapeutic effect of cevimeline on dry eye in patients with Sjögren's syndrome: a randomized, double-blind clinical study | journal = American Journal of Ophthalmology | volume = 138 | issue = 1 | pages = 6–17 | date = July 2004 | pmid = 15234277 | doi = 10.1016/j.ajo.2004.02.010 }}</ref><ref name="Fox_2019">{{cite book | vauthors = Fox RI, Fox CM | chapter = Management of Sjögren's |title=Dubois' Lupus Erythematosus and Related Syndromes|publisher=] |year=2019 |isbn=978-0-323-47927-1|edition=9th|pages=745-758|language=en |doi=10.1016/B978-0-323-47927-1.00060-8}}</ref> and ].<ref name="Ono_2004" /> It increases the production of saliva.<ref name="Fox_2019" /> | ||
| == Side effects == | == Side effects == | ||
Revision as of 21:15, 22 October 2023
Pharmaceutical drug Pharmaceutical compound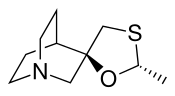 | |
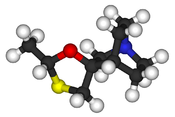 | |
| Clinical data | |
|---|---|
| Trade names | Evoxac |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608025 |
| Pregnancy category |
|
| Routes of administration | By mouth (capsules) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | <20% |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H17NOS |
| Molar mass | 199.31 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Cevimeline (trade name Evoxac) is a synthetic analog of the natural alkaloid muscarine with a particular agonistic effect on M1 and M3 receptors. It is used in the treatment of dry mouth and Sjögren's syndrome.
Medical uses
Cevimeline is used in the treatment of xerostomia (dry mouth) and Sjögren's syndrome. It increases the production of saliva.
Side effects
Known side effects include nausea, vomiting, diarrhea, excessive sweating, rash, headache, runny nose, cough, drowsiness, hot flashes, blurred vision, and difficulty sleeping.
Contraindications include asthma and angle closure glaucoma.
Mechanism of action
Cevimeline is a cholinergic agonist. It has a particular effect on M1 and M3 receptors. By activating the M3 receptors of the parasympathetic nervous system, cevimeline stimulates secretion by the salivary glands, thereby alleviating dry mouth.
See also
- Pilocarpine — a similar parasympathomimetic medication for dry mouth (xerostomia)
- Bethanechol — a similar muscarinic parasympathomimetic with longer-lasting effect
References
- ^ Ono M, Takamura E, Shinozaki K, Tsumura T, Hamano T, Yagi Y, Tsubota K (July 2004). "Therapeutic effect of cevimeline on dry eye in patients with Sjögren's syndrome: a randomized, double-blind clinical study". American Journal of Ophthalmology. 138 (1): 6–17. doi:10.1016/j.ajo.2004.02.010. PMID 15234277.
- ^ Fox RI, Fox CM (2019). "Management of Sjögren's". Dubois' Lupus Erythematosus and Related Syndromes (9th ed.). Elsevier. pp. 745–758. doi:10.1016/B978-0-323-47927-1.00060-8. ISBN 978-0-323-47927-1.
- "Cevimeline". MedicineNet. Retrieved 12 October 2007.