| |
| Names | |
|---|---|
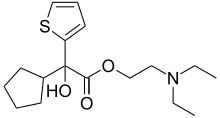
| Preferred IUPAC name 2-(Diethylamino)ethyl cyclopentyl(hydroxy)(thiophen-2-yl)acetate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.035.835 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C17H27NO3S |
| Molar mass | 325.47 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
WIN-2299 is an anticholinergic drug. Human reactions to WIN-2299 include sedation (2 mg), LSD-like reactions (6 mg), and an acute delirious episode (10 mg).
References
- Luduena, FP; Lands, AM (1954). "An investigation of the pharmacological actions of three potent antispasmodic compounds and their corresponding metho-salts". The Journal of Pharmacology and Experimental Therapeutics. 110 (3): 282–92. PMID 13143475.
- Pennes, HH; Hoch, PH (1957). "Psychotomimetics, clinical and theoretical considerations: Harmine, Win-2299 and nalline". The American Journal of Psychiatry. 113 (10): 887–92. doi:10.1176/ajp.113.10.887. PMID 13402982.
| Muscarinic acetylcholine receptor modulators | |||||
|---|---|---|---|---|---|
| mAChRsTooltip Muscarinic acetylcholine receptors |
| ||||
| Precursors (and prodrugs) | |||||
This hallucinogen-related article is a stub. You can help Misplaced Pages by expanding it. |