| |

| |
| Names | |
|---|---|
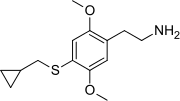
| Preferred IUPAC name 2-{4--2,5-dimethoxyphenyl}ethan-1-amine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H21NO2S |
| Molar mass | 267.39 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
2C-T-8 is a psychedelic phenethylamine of the 2C family. It was first synthesized by Alexander Shulgin, sometimes used as an entheogen.
Chemistry
The full name of the chemical is 2,5-dimethoxy-4-cyclopropylmethylthiophenethylamine. The compound is reported to have a bad taste and smell.
Effects
In his book PiHKAL, Shulgin lists the dosage range as 30 to 50 mg. 2C-T-8 is generally taken orally and effects typically last 10 to 15 hours. Experiences have varied between insight and creativity at low doses to hypersensitivity and paranoia at higher doses. A "thinking-connection" that is characteristic of the 2C-T group is evident in this chemical in stark contrast to the "pure euphoria" of phenethylamines such as MDMA.
Legality
2C-T-8 is unscheduled and uncontrolled in the United States, but possession and sales of 2C-T-8 will probably be prosecuted under the Federal Analog Act because of its structural similarities to 2C-T-7. However, 2C-T-8, unlike many other phenethylamines has not been sold by internet retailers. In the wake of Operation Web Tryp in July 2004, the issue of possession and sales of 2C-T-8 and other similar chemicals will probably be resolved in the courtroom as will the fate of this rare but unique psychedelic.
Canada
As of October 31, 2016, 2C-T-8 is a controlled substance (Schedule III) in Canada.
Pharmacology
| This article possibly contains original research. Please improve it by verifying the claims made and adding inline citations. Statements consisting only of original research should be removed. (September 2019) (Learn how and when to remove this message) |
The mechanism that produces 2C-T-8's hallucinogenic and entheogenic effects has not been specifically established, however it is most likely to result from action as a 5-HT2A serotonin receptor agonist in the brain, a mechanism of action shared by all of the hallucinogenic tryptamines and phenethylamines for which the mechanism of action is known.
Dangers
The toxicity of 2C-T-8 is not well documented. 2C-T-8 is somewhat less potent than 2C-T-7, but it may be expected that at higher doses it would display similar toxicity to that of other phenethylamines of the 2C-T family.
There have been no confirmed deaths due to 2C-T-8, though this may in part be due to its rarity and limited usage. Of the 2C-T family, there have been a few confirmed deaths due to 2C-T-7, which involved either insufflating large (>30 mg) doses and in one case an unknown oral dose was combined with 200 mg MDMA.
Popularity
2C-T-8 is unknown on the black market. Limited accounts of 2C-T-8 can be found in the book PiHKAL.
References
- ^ Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628. 2C-T-8 Entry in PiHKAL
- "Canada Gazette – Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)". 4 May 2016.
- Erowid.org
- Erowid.org
- Erowid.org