| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 2-ethan-1-amine | |
| Other names
2-ethan-1-amine 2-ethanamine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.215.648 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
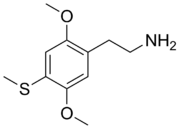
| Chemical formula | C11H17NO2S |
| Molar mass | 227.32 g/mol |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
2C-T (or 4-methylthio-2,5-DMPEA) is a psychedelic and hallucinogenic drug of the 2C family. It is used by some as an entheogen. It has structural and pharmacodynamic properties similar to the drugs mescaline and 2C-T-2.
It was first synthesized and studied through a collaboration between David E. Nichols and Alexander Shulgin.
Chemistry
2C-T is in a class of compounds commonly known as phenethylamines, and is the 4-methylthio analogue of 2C-O, a positional isomer of mescaline. It is also the 2C analog of Aleph. The systematic name of the chemical is 2-(2,5-dimethoxy-4-(methylthio)phenyl)ethanamine. The CAS number of 2C-T is 61638-09-3.
Effects
2C-T's active dosage is around 75–150 mg and produces mescaline and MDMA-like effects that may last up to 6 hours.
Pharmacology
The mechanism that produces 2C-T’s hallucinogenic and entheogenic effects has not been specifically established, however it is most likely to result from action as a 5-HT2A serotonin receptor agonist in the brain, a mechanism of action shared by all of the hallucinogenic tryptamines and phenethylamines for which the mechanism of action is known.
Popularity
2C-T is almost unknown on the black market although it has rarely been sold by "research chemical" companies. Limited accounts of 2C-T can be found in the book PiHKAL.
Legality
Canada
As of October 31, 2016; 2C-T is a controlled substance (Schedule III) in Canada.
United States
2C-T is unscheduled and unregulated in the United States; however its close similarity in structure and effects to 2C-T-7 could potentially subject possession and sale of 2C-T to prosecution under the Federal Analog Act. This seems to be the tack the federal government is taking in the wake of the DEA's Operation Web Tryp. A series of court cases in the US involving the prosecution of several online vendors were commenced in 2004 and resulted in a number of convictions.
See also
References
- Nichols DE, Shulgin AT (October 1976). "Sulfur analogs of psychotomimetic amines". J Pharm Sci. 65 (10): 1554–6. CiteSeerX 10.1.1.687.8486. doi:10.1002/jps.2600651040. PMID 978423.
- ^ Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628. 2C-T Entry in PiHKAL
- "Canada Gazette – Regulations Amending the Food and Drug Regulations (Part J — 2C-phenethylamines)". 4 May 2016.
- "Erowid Psychoactive Vaults : Research Chemicals : DEA Announces Arrests and Investigation, July 22, 2004".
| Phenethylamines | |
|---|---|
| Phenethylamines |
|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|