 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Catovit, Katovit, Promotil, Villescon |
| Routes of administration | By mouth, intranasal, rectal |
| Drug class | Stimulant; Norepinephrine–dopamine reuptake inhibitor (NDRI) |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.077 |
| Chemical and physical data | |
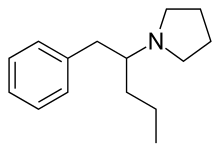
| Formula | C15H23N |
| Molar mass | 217.356 g·mol |
| 3D model (JSmol) | |
| Melting point | 133 °C (271 °F) |
| Boiling point | 153 °C (307 °F) |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Prolintane is a central nervous system (CNS) stimulant and norepinephrine–dopamine reuptake inhibitor (NDRI) developed in the 1950s. Being an amphetamine derivative, it is closely related in chemical structure to other drugs such as pyrovalerone, MDPV, and propylhexedrine, and has a similar mechanism of action. Many cases of prolintane abuse have been reported.
Under the brand name Katovit, prolintane was commercialized by the Spanish pharmaceutical company FHER until 2001. It was most often used by students and workers as a stimulant to provide energy and increase alertness and concentration.
See also
- α-PVP (β-ketone-prolintane, prolintanone)
- Methylenedioxypyrovalerone (MDPV)
- Pyrovalerone (Centroton, Thymergix)
- Phenylpropylaminopentane (PPAP)
References
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- Hollister LE, Gillespie HK (March–April 1970). "A new stimulant, prolintane hydrochloride, compared with dextroamphetamine in fatigued volunteers". The Journal of Clinical Pharmacology and the Journal of New Drugs. 10 (2): 103–9. doi:10.1177/009127007001000205. PMID 4392006.
- GB Patent 807835
- Nicholson AN, Stone BM, Jones MM (November 1980). "Wakefullness and reduced rapid eye movement sleep: studies with prolintane and pemoline". British Journal of Clinical Pharmacology. 10 (5): 465–72. doi:10.1111/j.1365-2125.1980.tb01790.x. PMC 1430138. PMID 7437258.
- Kyle PB, Daley WP (September 2007). "Domestic abuse of the European rave drug prolintane". Journal of Analytical Toxicology. 31 (7): 415–8. doi:10.1093/jat/31.7.415. PMID 17725890.
| Phenethylamines | |
|---|---|
| Phenethylamines |
|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |