 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
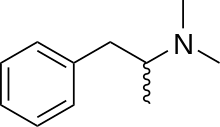
| Formula | C11H17N |
| Molar mass | 163.264 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Dimethylamphetamine (Metrotonin), also known as dimetamfetamine (INN), dimephenopan and N,N-dimethylamphetamine, is a stimulant drug of the phenethylamine and amphetamine chemical classes. Dimethylamphetamine has weaker stimulant effects than amphetamine or methamphetamine and is considerably less addictive and less neurotoxic compared to methamphetamine. However, it still retains some mild stimulant effects and abuse potential, and is a Schedule I controlled drug.
Dimethylamphetamine has occasionally been found in illicit methamphetamine laboratories, but is usually an impurity rather than the desired product. It may be produced by accident when methamphetamine is synthesised by methylation of amphetamine if the reaction temperature is too high or an excess of methylating agent is used.
It is said to be a prodrug of amphetamine/methamphetamine.
References
- Witkin JM, Ricaurte GA, Katz JL (May 1990). "Behavioral effects of N-methylamphetamine and N,N-dimethylamphetamine in rats and squirrel monkeys". The Journal of Pharmacology and Experimental Therapeutics. 253 (2): 466–74. PMID 2338643.
- Ricaurte GA, DeLanney LE, Irwin I, Witkin JM, Katz JL, Langston JW (June 1989). "Evaluation of the neurotoxic potential of N,N-dimethylamphetamine: an illicit analog of methamphetamine". Brain Research. 490 (2): 301–6. doi:10.1016/0006-8993(89)90247-3. PMID 2765865. S2CID 20682993.
- Fasciano J, Hatzidimitriou G, Yuan J, Katz JL, Ricaurte GA (October 1997). "N-methylation dissociates methamphetamine's neurotoxic and behavioral pharmacologic effects". Brain Research. 771 (1): 115–20. doi:10.1016/s0006-8993(97)00801-9. PMID 9383014. S2CID 8456534.
- Katz JL, Ricaurte GA, Witkin JM (1992). "Reinforcing effects of enantiomers of N,N-dimethylamphetamine in squirrel monkeys". Psychopharmacology. 107 (2–3): 315–8. doi:10.1007/BF02245154. PMID 1615131. S2CID 24707878.
- "Microgram Bulletin" (PDF). US Drug Enforcement Administration. Archived from the original (PDF) on 2013-04-14. Retrieved 2013-08-06.
- "The Identification of d-N,N-Dimethylamphetamine (DMA) in an Exhibit in Malaysia" (PDF). US Drug Enforcement Administration.
- Dettmeyer R, Verhoff MA, Schütz HF (9 October 2013). Forensic Medicine: Fundamentals and Perspectives. Springer Science & Business Media. pp. 519–. ISBN 978-3-642-38818-7.
| Phenethylamines | |
|---|---|
| Phenethylamines |
|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|