This is an old revision of this page, as edited by Edgar181 (talk | contribs) at 12:14, 21 February 2012 (Hill notation for organic compound). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 12:14, 21 February 2012 by Edgar181 (talk | contribs) (Hill notation for organic compound)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)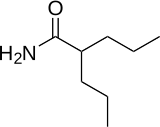
| |
| Names | |
|---|---|
| IUPAC name 2-Propylpentanamide | |
| Other names Depamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.017.632 |
| EC Number |
|
| KEGG | |
| MeSH | dipropylacetamide |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H17NO |
| Molar mass | 143.230 g·mol |
| Appearance | White crystals |
| Melting point | 125 °C (257 °F; 398 K) |
| log P | 2.041 |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
|
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Valpromide (marketed as Depamide by sanofi-aventis) is a carboxamide derivative of valproic acid used in the treatment of epilepsy and some affective disorders. It is rapidly metabolised (80%) to valproic acid (another anticonvulsant) but has anticonvulsant properties itself. It may produce more stable plasma levels than valproic acid or sodium valproate and may be more effectively at preventing febrile seizures. However it is over one hundred times more potent inhibitor of liver microsomal epoxide hydrolase. This makes it incompatible with carbamazepine and can affect the ability of the body to remove other toxins. Valpromide is no safer during pregnancy than valproic acid.
Valpromide is formed through the reaction of valproic acid and ammonia via an intermediate acid chloride.
In pure form, valpromide is a white crystalline powder and has melting point 125–126 °C. It is practically insoluble in water but soluble in hot water. It is available on the market in some European countries.
See also
Notes
- "dipropylacetamide - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 24 June 2005. Identification and Related Records. Retrieved 21 February 2012.
References
- The Medical Treatment of Epilepsy by Stanley R Resor. Published by Marcel Dekker (1991). ISBN 0-8247-8549-5.
- Hydrolysis in Drug and Prodrug Metabolism: Chemistry, Biochemistry, and Enzymology by Bernard Testa, Joachim M. Mayer (2003). ISBN 3-906390-25-X.
- In Vitro Methods in Developmental Toxicology by Gary L Kimmel, Devendra M Kochhar, Baumann (1989). ISBN 0-8493-6919-3.
| GABA receptor modulators | |||||
|---|---|---|---|---|---|
| Ionotropic |
| ||||
| Metabotropic |
| ||||
This anticonvulsant-related article is a stub. You can help Misplaced Pages by expanding it. |