This is an old revision of this page, as edited by Fonsy74 (talk | contribs) at 21:20, 9 March 2016 (Target receptor information with sources.). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
Revision as of 21:20, 9 March 2016 by Fonsy74 (talk | contribs) (Target receptor information with sources.)(diff) ← Previous revision | Latest revision (diff) | Newer revision → (diff)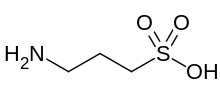
| |

| |
| Names | |
|---|---|
| IUPAC name 3-Aminopropane-1-sulfonic acid | |
| Other names Tramiprosate; Alzhemed; 3-APS | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.020.889 |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H9NO3S |
| Molar mass | 139.17 g·mol |
| Melting point | 293 °C (559 °F; 566 K) (decomposition) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Homotaurine (3-amino-1-propanesulfonic acid (3-APS) or tramiprosate (INN)) is a synthetic organic compound. It is analogous to taurine, but with an extra carbon in its chain. Because of its similarity in structure to the neurotransmitter gamma-aminobutyric acid (GABA), it has GABAergic effects and may be useful as an anticonvulsant.
It is a GABAB receptor antagonist and reversed the catatonia induced by baclofen in rats. Moreover, Homotaurine suppress ethanol-stimulated dopamine release, ethanol intake and preference in rats similar to acamprosate (Acetylated homotaurine analogue) .
Homotaurine is a zwitterion at neutral pH.

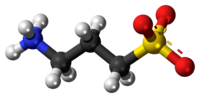 The zwitterionic form of homotaurine
The zwitterionic form of homotaurine
See also
References
- Homotaurine at Sigma-Aldrich
- Fariello RG, Golden GT, Pisa M; Golden; Pisa (1982). "Homotaurine (3 aminopropanesulfonic acid; 3APS) protects from the convulsant and cytotoxic effect of systemically administered kainic acid". Neurology. 32 (3): 241–5. doi:10.1212/wnl.32.3.241. PMID 7199633.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Giotti A, Luzzi S, Spagnesi S, Zilletti L. "Homotaurine: a GABAB antagonist in guinea-pig ileum". PMID 6652358.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - Mehta AK1, Ticku MK. "Baclofen induces catatonia in rats". PMID 2823166.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: numeric names: authors list (link) - Olive MF, Nannini MA, Ou CJ, Koenig HN, Hodge CW. "Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release". PMID 11864639.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link)
| GABA receptor modulators | |||||
|---|---|---|---|---|---|
| Ionotropic |
| ||||
| Metabotropic |
| ||||
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |