 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
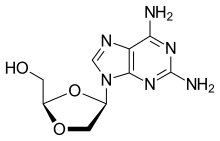
| Formula | C9H12N6O3 |
| Molar mass | 252.234 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Amdoxovir is a pharmaceutical drug that has undergone research for the treatment of HIV/AIDS. It acts as a nucleoside reverse transcriptase inhibitor (NRTI). The drug was discovered by Raymond F. Schinazi (Emory University) and C.K. Chu (University of Georgia) and developed by RFS Pharma.
Amdoxovir was in advanced Phase II clinical trials around 2010. In 2013, a Phase II trial was terminated and another was withdrawn before it started. No further studies appear to have been done.
References
- "Amdoxovir". AIDSmeds.com. January 13, 2009. Archived from the original on 2008-03-21. Retrieved March 21, 2008.
- Murphy RL, Kivel NM, Zala C, Ochoa C, Tharnish P, Mathew J, Pascual ML, Schinazi RF (2010). "Antiviral activity and tolerability of amdoxovir with zidovudine in a randomized double-blind placebo-controlled study in HIV-1-infected individuals". Antivir Ther. 15 (2): 185–192. doi:10.3851/IMP1514. PMC 7733239. PMID 20386073.
- Clinical trial number NCT01737359 for "A Safety and Efficacy Study of Amdoxovir in HIV-1 Treatment-experienced Subjects" at ClinicalTrials.gov
- Clinical trial number NCT01738555 for "A Safety and Efficacy Study of Amdoxovir in HIV-1 Treatment-experienced Subjects" at ClinicalTrials.gov
This antiinfective drug article is a stub. You can help Misplaced Pages by expanding it. |