 MK-2048 MK-2048 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.233.568 |
| Chemical and physical data | |
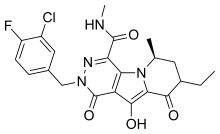
| Formula | C21H21ClFN5O4 |
| Molar mass | 461.88 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
MK-2048 is the Merck & Co. designation for a molecule in its pre-clinical drug discovery portfolio that is an integrase inhibitor-class of agent selected for development as a preventative treatment against HIV infection. Its second generation integrase design was hypothesized to be superior to the first available integrase inhibitor, raltegravir, in that "MK-2048 has a dissociation half-life of 32 hours on wild-type integrase—more than four times that of raltegravir", and its dissociation half-life against the important HIV integrase mutant N155H was on the same order of magnitude as that of raltegravir against wild-type virus. These findings led Merck representatives to suggest the possibility of "reduced susceptibility to resistance mutations" for the second generation drug. MK-2048 has been investigated for use as part of a pre-exposure prophylaxis (PrEP) approach to the treatment of HIV infection; however, the results of a 2015-2016 placebo-controlled human clinical trial indicated no observed correlation between tissue-associated VCV and/or MK-2048 and the inhibition of HIV infection, limiting expectations for this compound's efficacy for such applications. At the time of these reports, there was no indication of the time by which "MK-2048, or related compounds, be ready for clinical trials".
References
- ^ Mascolini M (April 2009). Conference Reports for NATAP: Merck Offers Unique Perspective on Second-Generation Integrase Inhibitor. 10th International Workshop on Clinical Pharmacology of HIV Therapy. Amsterdam]: NATAP.org. Archived from the original on June 4, 2017. Retrieved November 8, 2009.
- Grobler JA, McKenna PM, Ly S, Stillmock K, Bahnck C, Danovich RM, et al. (April 2009). Presentation, Abstract O-10: Functionally Irreversible Inhibition of Integration by Slowly Dissociating Strand Transfer Inhibitors. 10th International Workshop on Clinical Pharmacology of HIV Therapy. Amsterdam]: NATAP.org.
- Alcorn K (April 28, 2009). "Ralvetgravir Shows Potential for use as PrEP Drug". AIDSmap.com. Archived from the original on January 3, 2010. Retrieved November 8, 2009.
- Clinical trial number NCT02356302 for "Safety and Pharmacokinetics of Intravaginal Rings Containing Vicriviroc (MK-4176) and/or MK-2048" at ClinicalTrials.gov
- Hoesley CJ, Chen BA, Anderson PL, Dezzutti CS, Strizki J, Sprinkle C, et al. (March 2019). "Phase 1 Safety and Pharmacokinetics Study of MK-2048/Vicriviroc (MK-4176)/MK-2048A Intravaginal Rings". Clinical Infectious Diseases. 68 (7): 1136–1143. doi:10.1093/cid/ciy653. PMC 6424075. PMID 30289435.
This antiinfective drug article is a stub. You can help Misplaced Pages by expanding it. |