Chemical compound Pharmaceutical compound
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
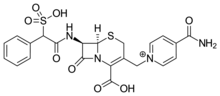
| Formula | C22H21N4O8S2 |
| Molar mass | 533.55 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Cefsulodin is a third-generation cephalosporin antibiotic that is active against Pseudomonas aeruginosa and was discovered by Takeda Pharmaceutical Company in 1977.
TAP Pharmaceuticals had a new drug application on file with FDA for cefsulodin under the brand name Cefonomil as of 1985.
Cefsulodin is most commonly used in cefsulodin-irgasan-novobiocin agar to select for Yersinia microorganisms. This agar is most often used in water and beverage testing.
Susceptibility data
The following represents MIC susceptibility data for various P. aeruginosa strains:
- Pseudomonas aeruginosa PA13 (resistant strain): 32 μg/ml
- Pseudomonas aeruginosa (wild-type, susceptible): 4 - 8 μg/ml
References
- "TOKU-E Technical Application Sheet" (PDF). Archived from the original (PDF) on 27 September 2011.
- Smith BR (1984). "Cefsulodin and ceftazidime, two antipseudomonal cephalosporins". Clinical Pharmacy. 3 (4): 373–85. PMID 6380902.
- Neu HC, Scully BE (1984). "Activity of cefsulodin and other agents against Pseudomonas aeruginosa". Reviews of Infectious Diseases. 6 (Suppl 3): S667–77. doi:10.1093/clinids/6.supplement_3.s667. PMID 6443768.
- Wright DB (November 1986). "Cefsulodin". Drug Intelligence & Clinical Pharmacy. 20 (11): 845–9. doi:10.1177/106002808602001104. PMID 3536385. S2CID 243377437.
- "Lupron Is First Abbott-Takeda Product to Reach U.S. Market". Pink Sheet. 15 April 1985.
- "BAM Media M35: Cefsulodin-Irgasan Novobiocin Agar or Yersinia Selective Agar". Food and Drug Administration. Retrieved 2 September 2012.
- "Cefsulodin | the Antimicrobial Index Knowledgebase - TOKU-E".