Pharmaceutical compound
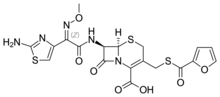 | |
| Clinical data | |
|---|---|
| Trade names | Naxcel |
| AHFS/Drugs.com | International Drug Names |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H17N5O7S3 |
| Molar mass | 523.55 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Ceftiofur is an antibiotic of the cephalosporin type (third generation), licensed for use in veterinary medicine. It was first described in 1987. It is marketed by pharmaceutical company Zoetis as Excenel, Naxcel, and Excede and is also the active ingredient in that company's Spectramast LC (lactating cow formulation) and Spectramast DC (dry cow formulation) product.
It is resistant to the antibiotic resistance enzyme beta-lactamase, and has activity against both Gram-positive and Gram-negative bacteria. Escherichia coli strains resistant to ceftiofur have been reported.
The metabolite desfuroylceftiofur also has antibiotic activity. The two compounds are measured together to measure for antibiotic activity in milk (alongside other antibiotics).
References
- "Naxcel EPAR". European Medicines Agency. 20 October 2009. Retrieved 29 June 2024.
- "Naxcel PI". Union Register of medicinal products. 24 May 2005. Retrieved 26 December 2024.
- Yancey RJ, Kinney ML, Roberts BJ, Goodenough KR, Hamel JC, Ford CW (1987). "Ceftiofur sodium, a broad-spectrum cephalosporin: evaluation in vitro and in vivo in mice". Am. J. Vet. Res. 48 (7): 1050–3. PMID 3631686.
- "Pfizer Animal Health Dairy Information on Products and Solutions". Archived from the original on 13 October 2007. Retrieved 20 November 2007.
- Donaldson SC, Straley BA, Hegde NV, Sawant AA, DebRoy C, Jayarao BM (2006). "Molecular epidemiology of ceftiofur-resistant Escherichia coli isolates from dairy calves". Appl. Environ. Microbiol. 72 (6): 3940–8. Bibcode:2006ApEnM..72.3940D. doi:10.1128/AEM.02770-05. PMC 1489609. PMID 16751500.
- Salmon SA, Watts JL, Yancey RJ (July 1996). "In vitro activity of ceftiofur and its primary metabolite, desfuroylceftiofur, against organisms of veterinary importance". Journal of Veterinary Diagnostic Investigation. 8 (3): 332–336. doi:10.1177/104063879600800309. PMID 8844576.
- "BetaStar Plus / For beta-lactam antibiotics / Product information sheet" (PDF). Neogen. Retrieved 9 September 2014.
This systemic antibiotic-related article is a stub. You can help Misplaced Pages by expanding it. |